
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Express the standard enthalpy of the reaction in 4 sig figs and include the appropriate units.
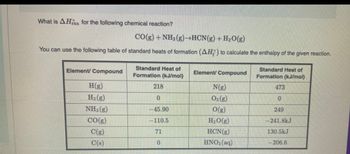
Transcribed Image Text:What is AH for the following chemical reaction?
CO(g) + NH3(g)→HCN(g) + H₂O(g)
You can use the following table of standard heats of formation (AH) to calculate the enthalpy of the given reaction.
Element/ Compound
H(g)
H₂(g)
NH3(g)
CO(g)
C(g)
C(s)
Standard Heat of
Formation (kJ/mol)
218
0
-45.90
-110.5
71
0
Element/Compound
N(g)
O₂(g)
O(g)
H₂O(g)
HCN (g)
HNO3(aq)
Standard Heat of
Formation (kJ/mol)
473
0
249
-241.8kJ
130.5kJ
-206.6
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 50-mL solution of a dilute AgNO3 solution is added to 100 mL of a base solution in a coffee-cup calorimeter. As Ag2O(s) precipitates, the temperature of the solution increases from 23.78 C to 25.19 C. Assuming that the mixture has the same specific heat as water and a mass of 150 g, calculate the heat q. Is the precipitation reaction exothermic or endothermic?arrow_forwardA 21.3-mL sample of 0.977 M NaOH is mixed with 29.5 mL of 0.918 M HCl in a coffee-cup calorimeter (see Section 6.6 of your text for a description of a coffee-cup calorimeter). The enthalpy of the reaction, written with the lowest whole-number coefficients, is 55.8 kJ. Both solutions are at 19.6C prior to mixing and reacting. What is the final temperature of the reaction mixture? When solving this problem, assume that no heat is lost from the calorimeter to the surroundings, the density of all solutions is 1.00 g/mL, the specific heat of all solutions is the same as that of water, and volumes are additive.arrow_forwardA 29.1-mL sample of 1.05 M KOH is mixed with 20.9 mL of 1.07 M HBr in a coffee-cup calorimeter (see Section 6.6 of your text for a description of a coffee-cup calorimeter). The enthalpy of the reaction, written with the lowest whole-number coefficients, is 55.8 kJ. Both solutions are at 21.8C prior to mixing and reacting. What is the final temperature of the reaction mixture? When solving this problem, assume that no heat is lost from the calorimeter to the surroundings, the density of all solutions is 1.00 g/mL, and volumes are additive.arrow_forward
- A sample of sucrose, C12H22O11, is contaminated by sodium chloride. When the contaminated sample is burned in a bomb calorimeter, sodium chloride does not burn. What is the percentage of sucrose in the sample if a temperature increase of 1.67C is observed when 3.000 g of the sample are burned in the calorimeter? Sucrose gives off 5.64103kJ/mol when burned. The heat capacity of the calorimeter and water is 22.51 kJ/C.arrow_forwardA 0.470-g sample of magnesium reacts with 200 g dilute HCl in a coffee-cup calorimeter to form MgCl2(aq) and H2(g). The temperature increases by 10.9 C as the magnesium reacts. Assume that the mixture has the same specific heat as water and a mass of 200 g. (a) Calculate the enthalpy change for the reaction. Is the process exothermic or endothermic? (b) Write the chemical equation and evaluate H.arrow_forwardAlloys When a 58.8-g piece of hot alloy is placed in125 g of cold water in a calorimeter, the temperature ofthe alloy decreases by 106.1°C, while the temperature ofthe water increases by 10.5°C. What is the specific heat ofthe alloy?arrow_forward
- In a coffee-cup calorimeter, 150.0 mL of 0.50 M HCI is added to 50.0 mL of 1.00 M NaOH to make 200.0 g solution at an initial temperature of 48.2C. If the enthalpy of neutralization for the reaction between a strong acid and a strong base is 56 kJ/mol, calculate the final temperature of the calorimeter contents. Assume the specific heat capacity of the solution is 4.184 J/g C and assume no heat Joss to the surroundings.arrow_forwardWhen solid iron burns in oxygen gas (at constant pressure) to produce Fe2O3(s), 1651 kJ of heat is released for every 4 mol of iron burned. How much heat is released when 10.3 g Fe2O3(s) is produced (at constant pressure)? What additional information would you need to calculate the heat released to produce this much Fe2O3(s) if you burned iron in ozone gas, O3(g), instead of O2(g)?arrow_forwardIn a bomb calorimeter, the reaction vessel is surrounded by water that must be added for each experiment. Since the amount of water is not constant from experiment to experiment, the mass of water must be measured in each case. The heat capacity of the calorimeter is broken down into two parts: the water and the calorimeter components. If a calorimeter contains 1.00 kg water and has a total heat capacity of 10.84 kJ/C, what is the heat capacity of the calorimeter components?arrow_forward
- In a calorimetric experiment, 6.48 g of lithium hydroxide, LiOH, was dissolved in water. The temperature of the calorimeter rose from 25.00C to 36.66C. What is H for the solution process? LiOH(s)Li(aq)+OH(aq) The heat capacity of the calorimeter and its contents is 547 J/C.arrow_forwardSalicylic acid, C7H6O3, is one of the starting materials in the manufacture of aspirin. When 1.00 g of salicylic acid burns in a bomb calorimeter, the temperature of the bomb and water goes from 23.11C to 28.91C. The calorimeter and water absorb 21.9 kJ of heat. How much heat is given off when one mole of salicylic acid burns?arrow_forwardOne step in the manufacturing of sulfuric acid is the conversion of SO2(g) to SO3(g). The thermochemical equation for this process is SO2(g)+12O2(g)SO3(g)H=98.9kJ The second step combines the SO3 with H2O to make H2SO4. (a) Calculate the enthalpy change that accompanies the reaction to make 1.00 kg SO3(g). (b) Is heat absorbed or released in this process?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co