
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
13. So it asap
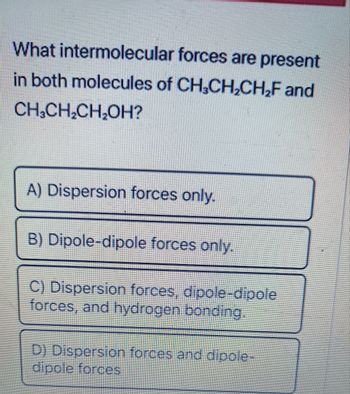
Transcribed Image Text:What intermolecular forces are present
in both molecules of CH₂CH₂CH₂F and
CH₂CH₂CH₂OH?
A) Dispersion forces only.
B) Dipole-dipole forces only.
C) Dispersion forces, dipole-dipole
forces, and hydrogen bonding.
D) Dispersion forces and dipole-
dipole forces
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- @ 79% 15:35 Wed 20 Jan T + : Note 20 Jan 2021 20 Jan 2021 at 15:34 The het Rer the electolytic prccoss is. equation Nat What anemical ameunt of electrons is requra te produce zmd of chiorine requriad te qas? 1 2 >arrow_forward5. A first order reaction is 75% complete in 58.0 seconds. How long will it take for to become 89.0% complete? At-0.25A0arrow_forwardDuring the following SEAr a rearrangementreaction can occur. Give the two possible productsand circle the product that will form preferentiallyarrow_forward
- m and 25°C. Is thi spontaneous at this (OEOTƏ, J/mol, AS = +38.5 J/K mo HV DMS DD 851.5-1\,L173 3. DD-DMS D7=84o.027 s the cròssover temperature for the reaction below? VH s) The decomposition of N20s is a first order reaction: 2N2O5 → 4NO2 + O2 The rate constant at a given temperature is found to be 5.25 x 10“ s'. If the initial concentration of N2O5 is 0.200 M, what is its concentration after exactly 10 minutes have passed? SHOW CH +SO CO 20 pies to findihe ethalny.of the abovarrow_forwardFast pls i will give u like for sure solve this question correctly in 5 min plsarrow_forwardPlease help me solve itarrow_forward
- Sign in Week 15 Recitation Caleb Young Oklahoma - CHEM 1315 (Section 001) - Fall20 - ROCHER > Activities and Due Dates > Week 15 Recitation O Assignment Score: 50% Resources O Hint Check Answer Question 9 of 10 > When methanol, CH, OH, is burned in the presence of oxygen gas, O,, a large amount of heat energy is released. For this 2: reason, it is often used as a fuel in high performance racing cars. The combustion of methanol has the balanced, thermochemical equation CH,OH(g) + 0,(g) → CO,(g) + 2H,O(1) AH = –764 kJ How much methanol, in grams, must be burned to produce 629 kJ of heat? 26.33 mass: TOOLS x10 about us careers privacy policy terms of use contact us help 5:14 PM 12/11/2020 50arrow_forward12:27 12-2 Analyzing Data... NAME Energy (kJ) NAME 1000 Many chemical reactions involve a change in energy. When the energy of the reactants is greater than the energy of the products, the reaction gives off heat and is exothermic. When the energy of the products is greater than that of the reactants, the reaction must absorb energy from the surroundings to occur and is endothermic. If the intermediate stage (the activated complex) is of a higher energy, then the reaction can only take place if the reactants are given an initial energy input (the activation energy). The graph shows energy diagrams that indicate how the energy changes as three reactions occur. Energy Changes for Three Reactions 800 600 Dashboard 400 200 DATE 0 ANALYZING DATA Interpret Energy Diagrams CLASS Direction of reaction 000 000 Calendar DATE .5GE 14 A D Copyright © Savvas Learning Company LLC. All Rights Reserved. Savvas is not responsible for any modifications made by end users to the content posted in its…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY