
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
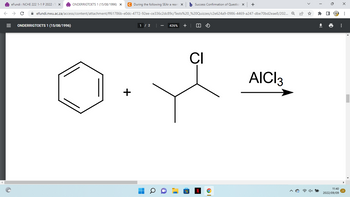
During the following SEAr a rearrangement
reaction can occur. Give the two possible products
and circle the product that will form preferentially
Transcribed Image Text:eFundi : NCHE 222 1-1 P 2022: T x
← → C
ONDERRIGTOETS 1 (15/08/1996) X C During the following SEAr a rear
efundi.nwu.ac.za/access/content/attachment/ff61786b-e0dc-4772-92ee-ce336c2dc89c/Tests%20_%20Quizzes/c2e624a9-0986-4469-a247-dbe70bd2eae8/202... Q
ONDERRIGTOETS 1 (15/08/1996)
+
1/2
■
b Success Confirmation of Question X +
426% +
H
CI
AICI 3
x
11:40
2022/09/09
⠀
⠀
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- = hrome File Edit View History Bookmarks Profiles Tab A ALEKS-Lara Althawadi -Lean X (129) ALEKS: Calculating the p X C www-awu.aleks.com/alekscgi/x/isl.exe/10_u-IgNsikr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInwFNs98WNYISBfkoq97Td3eiTwdipb... 4 Solubility and O O ACIDS AND BASES Calculating the pH at equivalence of a titration esc Try Again Your answer is incorrect. 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction pH=9.22 Explanation 30 Methods for Measuring the pli of an Aqueous Slide 28 of 104 Notes A chemist titrates 160.0 mL of a 0.5839 M benzoic acid (HC,H,CO₂) solution with 0.4219M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of benzoic acid is 4.20. Q Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. A Recheck 2 Comments W S X Click to add movers 3 P: ---+63% E 5 D (129) ALEKS:…arrow_forwardXA ALEKS-Artemio Ibarra- C www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNslkr7j8P3jH-fvgWyWxWinmDn7WsVrRAXK6XnHkiRvH2tl8oEjAi0u6py2zDxeBLth_JvbDwavi Content X (13) The Story Of Rock 'nx Content Module Knowledge Check An unknown compound has the following chemical formula: NO 0 Question 7 Launch Meeting - Zoom where x stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 8.7 mol of nitrogen and 4.3 mol of oxygen. Write the complete chemical formula for the unknown compound.arrow_forward||| Calend ? College AL X ChatGP P College Q percen I Apply Gaseou Central Richard (387) u Ask a C + C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IBIZZxiNVNkWhorBIB0d5GFRhLZ5YclOUixZOSyCPbErWEdQp9Q4Y170EfAPQIj... Q □ G O Chemical Reactions Reaction sequence stoichiometry 0/5 Matthe Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, and hydrogen prepared by reforming natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: N2(g) + 3H2(g) → 2 NH, (g) In the second step, ammonia and oxygen react to form nitric acid (HNO3) and water: NH2(g) + 202(g) → HNO3(g) + H2O (g) Suppose the yield of the first step is 62.% and the yield of the second step is 87.%. Calculate the mass of hydrogen required to make 10.0 kg of nitric acid. Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits. ☐ x10 Explanation Check OC 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy…arrow_forward
- ALEKS - Rafia Riaz - Learn G Some measurements of the initia X h ALEKS - Rafia Riaz - Learn M Gma x + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusK7wgZ0BOgkvzQMF9BNs_yz_5IM8YHDdX9aQSrX7x_QitSAMUvKq06T1iflMR?1... M Gmail YouTube Maps GTranslate News O KINETICS AND EQUILIBRIUM Calculating average and instantaneous reaction rate from a... Explanation Type here to search Here is a graph of the molarity of formic acid (HCO₂H) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. College information Check 3 발 0.30- 0.248 0.20- 0.15- 0.10 y 0.05+ 0 10 15 Is HCO₂H being created or destroyed by the chemical reaction? seconds 20 25 created O destroyed 30 X O neither created nor destroyed x10 00 8 9 0/3 ロ・ロ © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use (?) Privacy Center 29°F Clear ☆ Rafia ★ ES olo Ar Accessibility x Other bookmarks 10:30 PM 2/24/2023arrow_forwardDiscord | #-courtyar x y! mdc.edu - Yahoo Search Resu X V Official Miami Dade College X ← → C www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-UibwdleAUz2IYCcR4NM7yr_IpMriNwxObE8BNBktcRGBtTHK1XmgWvpNI8wuDkZtcqnDqEtC1XWgHvKTc... ☆ M Gmail ||| = YouTube OKINETICS AND EQUILIBRIUM Calculating the change in concentration after a whole number o... Translate V The rate at which a certain drug is eliminated by the body follows first-order kinetics, with a half life of 68 minutes. Round your answer to 2 significant digits. 82°F Partly sunny Hg mL Explanation Suppose in a particular patient the concentration of this drug in the bloodstream immediately after injection is 0.074 µg/mL. What will the concentration be 272 minutes later? Check X MHCampus/Connect(ALEKS) X A ALEKS-Mia Reboredo - Lear X Ś L Q Search + 1/5 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acce 四 e o 4) 5arrow_forwardSakai : CHM 151 170 FA21: Exan x -> b sakai.durhamtech.edu/portal/site/2021fa-chm-151-170/tool/5f0a6298-e555-44a9-9a8f-40c43992fa29/jsf/delivery/deliverAssessment Sakai : My Workspa. www.cfnc.org | 520. + Scholarships - Goo. C Going Merry - Brow.. A Gates: Sign Up wh ccsf Application Status f. A MyMountaineer E Reading list >> The pressure of carbon dioxide gas in a sealed 2.5 L flask is 2.1 atmospheres. Calculate the new pressure of carbon dioxide if all gas was transferred to a 7.5 L flask. OA 6.3 atm O B. You need to know the number of moles and the temperature of CO, to perform this calculation. OC 0.70 atm OD. 2.1 atm OE 4.2 atm Reset Selection Previous Next Save O EXTD V O 5:54 DELLarrow_forward
- alentina Vergara - Le X www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJ-TnplXoFu0F7UjsroJMKrbFHXPNvwILIE9eRim2d5l07ErwABTCdbvgUnFtjhtelPZIZL910... Homepage Welcome, Valentin... E Freeze | myEquifax EMT Fall 2022- M... gara, Val... Google Docs A solution is prepared at 25°C X + O ACIDS AND BASES Calculating the pH of a buffer pH = Explanation Check X S GOVT 2306.003:... A solution is prepared at 25 °C that is initially 0.30M in methylamine (CH3NH₂), a weak base with K-4.4 x 104, and 0.32M in methylammonium chloride (CH3NH₂C1). Calculate the pH of the solution. Round your answer to 2 decimal places. * ALEKS - Valentina... 0/5 B Health and sport... C Valentina V ©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility da A O ?arrow_forwardMALEKS T LI ALEKS - Rafia Riaz - Knowledge CX + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtHPKeXbFh_esrw9lm-BbWISQqFfd1OKKzQ55nmjVf-6W1kBQ5aycVAYBaQo?... Type here to search = Module Knowledge Check A chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 33.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor…arrow_forwardMALEKS T LI ALEKS - Rafia Riaz - Knowledge CX + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtHPKeXbFh_esrw9lm-BbWISQqFfd1OKKzQ55nmjVf-6W1kBQ5aycVAYBaQo?... Type here to search = Module Knowledge Check Read the descriptions of physical or chemical changes in the table below. Then decide whether the change will be spontaneous, if you can. A chemical reaction between two liquids creates gaseous products. A gas expands, absorbing heat from its surroundings. Change During an exothermic chemical reaction, a solid is consumed and a gas produced. I Don't Know Et Submit Question 1 3 Is this change spontaneous? Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given. O Yes. O No. O Can't decide with information given. X Rafia V ? 13 ollo © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 65°F Mostly clear (?) F * (P 9:08 PM 5/10/2023 x : 6arrow_forward
- ALEKS - Jacqueline Hoppenrey x G convert mg to g - Google Searc x A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQiHqRdYV 6Ux63Syp.JXz0Coxvwqgg4JkWI72X79QvOLp9_7U27sYQhkaocvdwecGvsUzo65uy3F6spORRg1XSqgh81is O STOICHIOMETRY Using molarity to find solute mass and solution volume Jacqueline A chemist adds 55.0 mL of a 4.75M silver perchlorate (AGCIO,) solution to a reaction flask. Calculate the mass in grams of silver perchlorate the chemist has added to the flask. Round your answer to 3 significant digits. Explanation Check Privacy Accessibil 2021 McGraw-Hill Education All Rights Reserved Terms of Usearrow_forwardCou X A Ch 6 x C Sear X * Sear X Sear x b > Hov x2 Con x Con X b Sear X y! cher X M Inbc X M Inbc X + Vent X 8 https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id3D15033867 + Sapling Learning macmillan learning Ch 6 Homework Jason Bauer , Sapling Learning > Ventura College - Chem V30 (31510) - Spring21 - ALAWDI > Activities and Due Dates > Ch 6 Homework E 20 of 24 Questions O Assignment Score: O Resources O Hint 68.3% Check Answer O 16 Question 100% x2 Question 20 of 24 > 1 of 5 Attempts Correct How many moles of CaCl2 are in 7.76 x 1024 formula units? O 17 Question 100% x2 1 of 5 Attempts Correct 7.76 x 1024 formula units = mol O 18 Question 100% x2 2 of 5 Attempts Correct O 19 Question 1 of 5 Attempts 100% x2 Correct 20 Question 0% O of 5 Attempts 21 Question 0% x2 O of 5 Attempts © 2011-2021 Sapling Learning, Inc. about us careers privacy policy terms of use contact us help 1:42 PM P Type here to search 99+ 2/18/2021 19arrow_forwardA ALEKS-Lara Althawadi O ☆ www-awu.aleks.com/alekscgi/x/isl.exe/10_u-IgNsikr7j8P3JH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInCwZsOvg6oljUPZmqCsVfxljXe3yeB7wt2E... a Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of.... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le... Math 115 W-S Fall.... h. Get star O ACIDS AND BASES Calculating the pH at equivalence of a titration pH = 0 A chemist titrates 120.0 mL of a 0.8655 M ammonia (NH3) solution with 0.7463M HBr solution at 25 °C. Calculate the pH at equivalence. The pK, of ammonia is 4.75. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. Explanation Check ethods for Measuring the pH of an Aqueous 28 of 104 Notes 2 x S Comments Click to add notes 3 E X :: D $ 4 C Q S > R F 5 I T + 63% G 6 53 B I'm Y 7 H Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY