
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What does this test indicate about the unknown molecule?
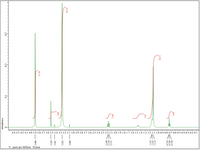
Transcribed Image Text:44 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 24 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.3 14 1.3 1.2 1.1 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1
| |
X: parts per Million : Proton
abundance
6 - 006'
21.26
3.529
3.441
3.265
49.52
- 880'E
2.189
L.155
1.143
L115
28.33
0.754
0.738
0.720
00 E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Formaldehyde, acetic acid, and glyceraldehyde are three molecules that share the same empirical formula (CH2O) but have different molecular formulas. Given the following information, what are the molecular formulas of these compounds? a) Formaldehyde molecules each contain 1 carbon atom. b) Acetic acid has a molar mass of 60.05 g/mol. c) Glyceraldehyde molecules each contain 6 hydrogen atoms. Criteria: I can explain the relationship between empirical formula and molecular formula with clarity and logic.arrow_forwardvalues needed for this question. 1. How many MOLECULES of disulfur decafluoride are present in 5.24 grams of this compound? 2. How many GRAMS of disulfur decafluoride are present in 7.04x1022 molecules of this compound? molecules. grams.arrow_forwarddetermine the number of atoms of 23.64g of Cearrow_forward
- (d) Write the names of the compounds below on the lines provided. ÇH3 H3C-N N. H;C CH-CH H,C CH HO. N. CH3 CH3 OH CH3 HO CH H.arrow_forwardDetermine the percent composition by mass of all elements in NCl5. And Determine the percent by mass of all elements in the compound C2H5O4. PLEASE SHOW ALL WORK!!!!! THANKS!arrow_forwardBr the proper name for these compounds: OH OH Br a) b) CI CI c) CI d) CH3 H3C. CH3 H3C Br онarrow_forward
- Question 30arrow_forwardCalculate the mass (in g) of 1.00 mol of C13H18O2 (ibuprofen). Show all steps of the calculations. (Please type answer no write by hend)arrow_forwardBl- II y Hydrate Experiment X C lab 10 empirical formula.pd X * Lab 11 - Atomic Fingerprints X G periodic table - Google Sear X C ALEKS ALEKS - Courtney Whiting + x i www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lijulplIKKWv4bwenmyrLYoBHbAAsJtmzj53jaq9t9R0TkuPCuyyRzYUtqxj0A3Nolvčdbxc4hZtxUYAFVf0BpHIpRqEr580?1oBw7QYjlbavbS.. O CHEMICAL REACTIONS Limiting reactants 1/5 Courtney V Liquid octane will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). Suppose 2.28 g of CH, octane is mixed with 6.0 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. OLX Explanation Check ©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use l Privacy Center Accessibilityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY