
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
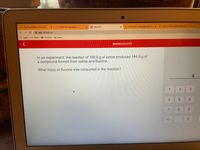
Transcribed Image Text:OSU MyOregonState - Overview
CHEM 101 Homework
10 Chem101
Inbox (59) - ketelk@oregonsta X
Week 1 Post-lab/Reading Quiz: x+
A app.101edu.co
Apps
M Gmail
O YouTube
News
Question 9.a of 49
In an experiment, the reaction of 100.0 g of iodine produced 144.9 g of
a compound formed from iodine and fluorine.
What mass of fluorine was consumed in the reaction?
2.
3
4.
7
8.
9.
+/-
LAir
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the mass in grams of 6.65 × 10²¹ atoms of barium. (The mass of one mole of barium is 137.33 g.)arrow_forwardWhat is the mass of 4.12 x 10^23 water molecules?arrow_forwardIn a combustion reaction, 30.8 g of ethanol reacts with 64.3 g of oxygen to produce water and carbon dioxide. If 36.2 g of water is produced, how much carbon dioxide is produced?arrow_forward
- 3.97 x 10^24 molecules of NO has what massarrow_forwardCalculate how many grams of chlorine gas are in 7.496 x 1023 atoms of chlorine gas.arrow_forwardIn a combustion reaction, 38.2 g of hydrocarbon reacts with 96.0 g of oxygen to produce water and carbon dioxide. If 53.8 g of water is produced, how much carbon dioxide is produced?arrow_forward
- How many milligrams can be found in a sample of tin (IV) phsophate that contains 8.7 x 10^22 atoms of oxygen?arrow_forwardthe mass of Cu (63.35 g/mol) used to create Cu2S was 41.95 grams. what theoretical mass of Cu2S (159.16 g/mol) can be produced?arrow_forward13.0 g of titanium reacted with 60.0 g of bromine. In this reaction, assuming 100% yield, 69.0 g of titanium(IV) bromide were produced and 4.01 g of titanium were left after the reaction. Which reactant was the limiting reactant? Which reactant was the excess reactant? Explain your reasoning using complete sentences.arrow_forward
- Ammonium perchlorate (NH4C104) is the solid rocket fuel that was used by the U.S. Space Shuttle and is used in the Space Launch System (SLS) of the Artemis rocket. It reacts with itself to produce nitrogen gas (N₂), chlorine gas (C1₂), oxygen gas (O₂), water (H₂O), and a great deal of energy. What mass of oxygen gas is produced by the reaction of 9.70 g of ammonium perchlorate? Round your answer to 3 significant digits. g x10 Xarrow_forwardWhen the supply of oxygen is limited, iron metal reacts with oxygen to produce a mixture of FeO and Fe2O3. In a certain experiment, 20.00 g iron metal was reacted with 11.48 g oxygen gas. After the experiment, the iron was totally consumed, and 3.26 g oxygen gas remained. Calculate the amounts of FeO and Fe2O3 formed in this experiment. A, Mass of FeO = B. Mass of Fe2O3 =arrow_forwardSmall amounts of platnium are used in automotive spark plugs. What is the mass, in grams, of 3.21 x 1020 atoms of platnium?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY