
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
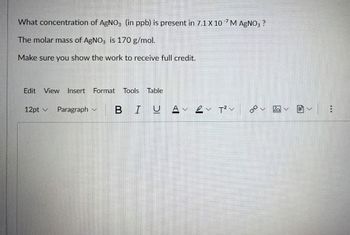
Transcribed Image Text:What concentration of AgNO3 (in ppb) is present in 7.1 X 10 M AgNO3 ?
The molar mass of AgNO3 is 170 g/mol.
Make sure you show the work to receive full credit.
Edit View Insert Format Tools Table
12pt ✓ Paragraph
BIUAv2v T²V
هم
阎圃v|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pls help ASAParrow_forwardAnalysis shows the 15.0 L of seawater simulant contains a mercury (II) ion concentration of 3 X 10-4 M. 1.494 grams of potassium iodide will be needed to add to the icefish tank to precipitate out all of the mercury (II) ions. The reaction of mercury (II) ions and potassium iodide is shown below:Hg22+(aq) + 2KI (s) Hg2I2(s) + 2K+(aq) What is the concentration of K+ions (in M) in the crocodile icefish tank after precipitating out all the mercury (II) ions?arrow_forwardDo not give handwriting solution.arrow_forward
- Consider the neutralization reaction 2 HNO, (aq)+Ba(OH), (aq) 2 H, O(1)+Ba(NO,),(aq) A 0.115 L sample of an unknown HNO, solution required 36.7 mL of 0.250 M Ba(OH), for complete neutralization. What is the concentration of the HNO, solution? concentration: M * TOOLS x10arrow_forwardesc Fr 2 What mass of precipitate is formed when 100. mL of 0.300 M NaCl reacts with 100. mL of 0.150 M Pb(NO3)? Round your answer to 3 significant digits. 55°F Clear 1 F1 B.D F2 @ 2 F3 3 E F4 LA 4 x10 Q Search F5 X % 5 S F6 C A 6 F7 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibil B 8 & 7 F8 * ос F9 DELL prt sc F10 home Submit Assignment F11 A end F12 insert + 5/arrow_forwardQuestion 9 of 21 > O Macmillan Learning You decide it is time to clean your pool since summer is quickly approaching. Your pool maintenance guide specifies that the chlorine, Cl₂, concentration of the pool should be between 1 and 3 ppm. In order to determine if your pool is safe to swim in, you send a sample of pool water to a chemist for analysis of the Cl₂ content. The chemist reports a chlorine concentration of 3.92 × 10-5 M. Convert the concentration of Cl₂ to parts per million (ppm). concentration: 2.71 Incorrect Attempt 3 ppmarrow_forward
- A solution contains 8.70×10-³ M lead nitrate and 1.47x10-² M barium acetate. Solid sodium phosphate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of phosphate ion when this precipitation first begins? [PO4³-] = M Submit Answer Retry Entire Group 1 more group attempt remainingarrow_forward(Multi answers) Find soluble compounds using the solubility rules. TABLE 9.7 Solubility Rules for lonic Compounds in Water An ionic compound is soluble in water if it contains one of the following: Li'. Na, K, Rb, Cs, NH₂ NO.C₂H₂O₂ CI. Br.I except when combined with Ag", Pb, or Hg²+ So except when combined with Ba, Pb, Ca, Sr, or Hg²+ Ionic compounds that do not contain at least one of these ions are usually insoluble. Positive lons Negative lons Select 5 correct answer(s) CuS KNO3 OKCI CaSO4 Na₂S AgCl AgNO3 RbBr BaSO4arrow_forwardPart A) A 10.0 mL sample of 0.250 mol/L NiF2(aq) is mixed with 20.0 mL of 0.0900 mol/L NaOH(aq) and then diluted to a final volume of 100. mL.Calculate the concentration of Ni2+ ions in the 100 mL mixture before the reaction starts. Express your answer to three significant figures.ksp: 5.48e-16arrow_forward
- Chemistry R120 Study Assignment A - General Solution Stoichiometry Problems a) Will all of the zinc dissolve? b) What will be the concentration of Br- in the resulting solution (after any reaction takes place)? 1. 18.0 g of zinc is added to 100. mL of 0.200M HBr. ****arrow_forward5:15 1 ull 4G Discussion Assignment #6 O o o INFO DISCUSSION Discussion Assignment #6 Due:Wednesday, 14 Oct 2020, 3:00 PM In titration, one solution is added to another solution until a chemical reaction between the components in the solutions has run to completion. The solution being added is called the titrant, and we say that it solution. The completion of the reaction is usually shown by a change of color caused by a substance called an indicator. used titrate the other A typical titration proceeds in the following way. A specific volume of the solution to be titrated is poured into an Erlenmeyer flask. For example, 15.0 mL of a sulfuric acid solution of unknown concentration is added to a 250 mL Erlenmeyer flask. An indicator is then added to the flask, which changes color when the reaction is complete. The titrant in this example is potassium hydroxide, having a concentration of 0.121 M. The titrant is added to a buret. After slowly adding the titrant to the flask, the reaction…arrow_forward13. Hydroxyapatite is the main mineral component of bone. How many grams of hydroxyapatite will dissolve in 1.00 L of H20? Ksp = 2.34 x 10-59; Molar mass = 502.3 g/mol Cas (PO4)30H (s) 5 Ca2+ (aq) + 3 PO43- (aq) + OH- (aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY