
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
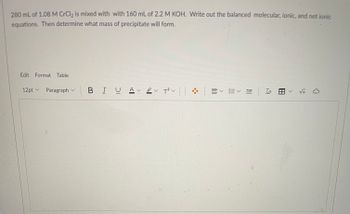
Transcribed Image Text:280 mL of 1.08 M CrCl₂ is mixed with with 160 mL of 2.2 M KOH. Write out the balanced molecular, ionic, and net ionic
equations. Then determine what mass of precipitate will form.
Edit Format Table
12pt Paragraph
V
BIU A v T²V |
V
E✓ ✓ To ✓ √x
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. potassium acetate and ammonium bromide zinc sulfate and sodium sulfide potassium chloride and silver nitratearrow_forwardAn aqueous solution of hydrobromic acid is standardized by titration with a 0.120 M solution of barium hydroxide.If 11.7 mL of base are required to neutralize 22.1 mL of the acid, what is the molarity of the hydrobromic acid solution?M hydrobromic acidarrow_forwardTo determine the molarity of an unknown sulfuric acid solution in a titration, a standardized NaOH solution with a molarity of 0.138 M was given. A student used 11.8 mL of the NaOH solution to reach to the end point of the titration with a 25.0 mL sample of the unknown acid solution. What is the molarity of the unknown sulfuric acid solution?arrow_forward
- Consider two aqueous solutions: one of ammonium carbonate and one of barium nitrate. List the ions present in each. Is a precipitate formed when the two solutions are mixed? If so, write the molecular, ionic and net ionic equation. Identify the spectator ionsarrow_forwardA 25.00 mL sample of sodium hydroxide was titrated (neutralized) with 36.4 mL of 0.125 M sulfuric acid. What is the concentration of the sodium hydroxide solution? Group of answer choices 0.364 M 0.728 M 1.46 M 0.091 M 0.182 Marrow_forwarda) The addition of 0.3800 L of 1.150 M KCl to a solution containing Ag+ and Pb2+ ions is just enough to precipitate all of the ions as AgCl and PbCl2. The total mass of the resulting precipitate is 61.60 g. Find the masses of PbCl2 and AgCl in the precipitate. b) A solution contains Al3+ and Co2+ The addition of 0.3533 L of 1.847 M NaOHNaOH results in the complete precipitation of the ions as Al(OH)3 and Co(OH)2. The total mass of the precipitate is 23.08 g Find the masses of Al3+ and Co2+ in the solution.arrow_forward
- 7) A sample of an unknown alkaline earth metal hydroxide is dissolved in 100.0 mL of water. The resulting solution is titrated to an indicator endpoint with 1.008 M aqueous All Sections b) If the sample amount is 1.042 g, what is the molar mass of the unknown metal hydrochloric acid. The indicator changes color after 17.00 mL of the acid has been added. a) Write the generic equation. hydroxide? hsoc) What is the identity of the metal?arrow_forwardComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B empirical formula of solution A solution B precipitate are mixed? olo manganese(II) iodide cadmium acetate O yes no silver nitrate sodium bromide yes no 18 Ar sodium sulfide zinc nitrate O yes noarrow_forwardTitration is a type of experiment that can be performed to investigate a neutralization reaction. The equivalence point is when all of the acid and base is fully neutralized. A sample of 0.869 M aqueous potassium hydroxide was titrated against a standard solution of hydrochloric acid. What was the volume of the potassium hydroxide solution if 77.5 mL of 1.50 M hydrochloric acid was needed to reach the equivalence point? Enter a numerical answer only, in terms of mL.arrow_forward
- Determining the volume of base needed to titrate a given mass of acid.arrow_forwardComplete and balance the precipitation reactions. Include physical states. Refer to the solubility rules as necessary. K3PO4(aq)+MgCl2(aq)arrow_forwardWhen excess lead(II) nitrate was added to 225 mL of a solution of potassium iodide, a solid precipitate formed. The dry precipitate had a mass of 7.25 g. What was the concentration of iodide ions in the solution of potassium iodide? a) 0.193 mol/L b) 0.140 mol/L c) 0.280 mol/L d) 0.070 mol/L e) 0.096 mol/Larrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY