
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
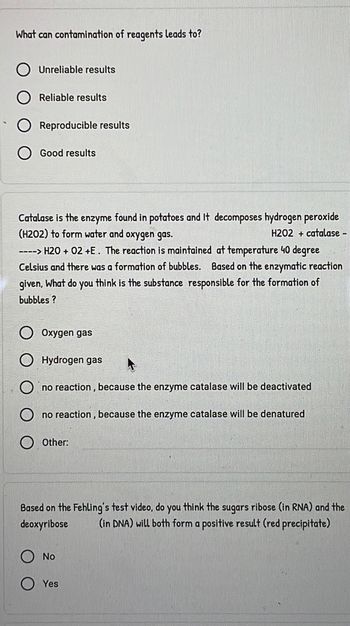
Transcribed Image Text:What can contamination of reagents leads to?
Unreliable results
Reliable results
Reproducible results
Good results
Catalase is the enzyme found in potatoes and It decomposes hydrogen peroxide
(H202) to form water and oxygen gas.
H202 + catalase -
----> H2O + 02 +E. The reaction is maintained at temperature 40 degree
Celsius and there was a formation of bubbles. Based on the enzymatic reaction
given, What do you think is the substance responsible for the formation of
bubbles?
Oxygen gas
Hydrogen gas
no reaction, because the enzyme catalase will be deactivated
no reaction, because the enzyme catalase will be denatured
Other:
Based on the Fehling's test video, do you think the sugars ribose (in RNA) and the
deoxyribose (in DNA) will both form a positive result (red precipitate)
No
Yes
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C 2 N₂O5 → 4NO2 + O2 the following data have been obtained: [N2O5], M 0.594 0.329 0.182 0.101 time, min 0 144 288 432 The average rate of disappearance of N₂O5 over the time period from t = 0 min to t = 144 min is M/minarrow_forwardThe rate of a certain reaction was studied at various temperatures. The table shows temperature (7) and rate constant (k) data collected during the experiments. Plot the data to answer the questions. What is the value of the activation energy, Ea, for this reaction? E₂ = What is the value of the pre-exponential factor (sometimes called the frequency factor), A, for this reaction? A = kJ. mol-¹ $ $1 T(K) 400 420 440 460 480 500 520 540 560 580 k (s-¹) 0.000369 0.00200 0.00928 0.0377 0.136 0.445 1.33 3.64 9.31 22.3arrow_forwardAmmonium ion reacts slowly with nitrite ion: NH4+ (aq) + NO2-(aq)-> N2(g) + 2H20 (1) Rate data for this reaction, measured at a certain temperature, are shown in the attached table. Use your rate law, and the rate and concentrations for any of the experiments, to calculate the rate constant (k) for the reaction in /Ms. Type your response: Expt. [NH4+] (M) [NO₂] (M) Rate (M s-1) 1 0.150 0.220 7.00 x 10-6 2 0.600 0.220 2.80 x 10-5 3 0.600 0.110 1.40 x 10-5arrow_forward
- |回|回|回一回|日 https://bbhosted.cuny.edu/webapps/assessment/take/launch.jsp?course assessment id3 2032637_1&course Remaining Time: 1 hour, 27 minutes, 03 seconds. * Question Completion Status: QUESTION 5 Which of the following statements about a catalyst is true? O Catalysts increase the rate of the reaction. O Catalysts increase the activation energy of a reaction. O Catalysts decrease the rate of the reaction. O Catalysts cause a system at equilibrium to shift towards the reactants. O Catalysts cause a system at equilibrium to shift towards the products. QUESTION 6 What is the hydronium (H2O*) concentration of a solution with a pH of 5.16? Click Save and Submit to save and submit. Click Save All Answers to save all ansuwers. here to search DELLarrow_forwardMethyl salicylate has the odor of oil of wintergreen. This ester is prepared by the Fisher esterification of salicylic acid with methanol, shown here: HSO, OH * CH,OH OCH, • H,0 +] OH OH Use the dropdowns below to indicate the order of the steps that occur in this reaction mechanism to transform salicylic acid into methyl salicylate. Step 1 [ Choose (Choose Step 2 Protonation of the C-O oxygen atom Step 3 Proton transfer to neutralize the product Reforming of the CHOand loss of the leaving group Step 4 Proton transferts the OH group on the tetrahedral intermediate Attack of the methanol on the carbon of the C-O Step 6 Breaking of the pi bond to push eléctrons onto exygeri nd form the tetrahedial intermedianarrow_forwardIdentify the stages of initiation, propagation, delay, inhibition, and termination in the following chain mechanism; If the stage does not exist, respond with a false, if it does exist, place a true. A. → B. + C A. + B. → P A2 → A. + A. A. + P→ B. Initiation: Propagation: Delay: Inhibition: Termination:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY