
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:- I need BOTH questions answered PLEASE!
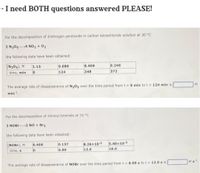
For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C
2 N2054 NO2 + 02
the following data have been obtained:
[N205), M
1.13
0.680
0.409
0.246
time, min
124
248
372
The average rate of disappearance of N20s over the time period from t= 0 min to t = 124 min is
M
min.
For the decomposition of nitrosyl bromide at 10 °C
2 NOBR2 NO + Br2
the following data have been obtained:
[NOBr), M
0.400
0.137
8.26x10-2
5.40x10 2
time, s
6.00
12.0
18.0
The average rate of disappearance of NOBR over the time period from t = 6.00 s to t = 12.0 s is
Ms1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C 2 N₂05- →4 NO₂ + O₂ the average rate of disappearance of N₂O5 over the time period from t = 0 min to t = 103 min is found to be 3.23x10-3 M min-¹. The average rate of formation of O₂ over the same time period is M min-¹.arrow_forwardFor the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C 2 N₂05- →4 NO₂ + O2 the average rate of disappearance of N₂05 over the time period from t = 0 min to t = 166 min is found to be 2.84x10-3 M min-¹. -1 The average rate of formation of O2 over the same time period is M min-¹.arrow_forwardThe following data are for the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C. N₂O5 →→→→→2 NO₂ + ½ 02 [N₂05 ], M 0.200 0.100 5.00×10-2 time, min 0 133 266 Hint: It is not necessary to graph these data. (1) The half life observed for this reaction is 133 (2) Based on these data, the rate constant for this reaction is min -1 min. order 2.50×10-2 399arrow_forward
- For the gas phase decomposition of sulfuryl chloride at 600 K SO,Cl,- →SO2 + Cl2 the following data have been obtained: [SO,Cl2], M 1.62x10-3 340 4.20×10-3 2.61x10-3 1.01x103 time, min 170 510 The average rate of disappearance of SO,Cl2 over the time period from t= 0 min to t= 170 min is M min!.arrow_forwardIn a study of the decomposition of hydrogen iodide on a gold surface at 150 °C HI(g) ½ H2(g) + ½ I½(g) the following data were obtained: [HI], M 0.509 0.255 0.128 6.40×10-2 seconds 520 780 911 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.509 M is s and when the starting concentration is 0.255 M is (2) The average rate of disappearance of HI from t= 0 s to t= 520 s is Ms (3) The average rate of disappearance of HI from t= 520 s to t= 780 s is Ms! (4) order reaction is Ms1 Based on these data, the rate constant for thisarrow_forwardFor the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 °C 2 N½O54 NO2 + O2 the following data have been obtained: N2O5], M 0.843 0.513 0.313 0.190 time, min 121 242 363 The average rate of disappearance of N205 over the time period from t= 242 min to t= 363 min is M minarrow_forward
- For the decomposition of nitrous oxide at 565 °C 2 N20 2 N2 + 02 the average rate of disappearance of N20 over the time period from t 0 s to t= 1088 s is found to be 5.45×104 Ms. The average rate of formation of O, over the same time period is Ms.arrow_forwardFor the gas phase decomposition of hydrogen peroxide at 400 °C 2 H2O2 2 H2O + O2 the following data have been obtained: [H,O2], M 9.35x10-2 3.54x10-2 2.18×10-2 1.56x10-2 time, s 27.0 54.0 81.0 The average rate of disappearance of H,O2 over the time period from t = 27.0 s to t = 54.0 s is |Ms-!.arrow_forwardIn a study of the decomposition of hydrogen iodide on a gold surface at 150 °C HI(g)% H,(g) + % »(9) the following data were obtained: HI], M seconds 0.274 0.137 6.85×10-2 3.43x10-2 521 782 912 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.274 M is s and when the starting concentration is 0.137 M is (2) The average rate of disappearance of HI from t= 0s to t= 521 s is| Ms!. (3) The average rate of disappearance of HI from t= 521 s to t= 782 s is| Ms! (4) Based on these data, the rate constant for this | | order reaction is Ms!.arrow_forward
- For the gas phase decomposition of phosphine at 120 °C 4 PH3 (9) → P4 (9)+ 6 H₂ (9) the following data have been obtained: [PH3], M time, s 0 The average rate of disappearance of PH3 over the time period from t = 0s to t = 23.0 s is 0.0644 0.0426 0.0281 0.0186 23.0 46.0 69.0 M/s.arrow_forward11). The hydrolysis (the degradation) of maltose (a type of sugar) is monitored with respect to time. The data collected for this experiment are as shown in the table below. The degradation reaction is: Maltose glucose + glucose Time, (min) Maltose, M 0.316 0.274 39 80 140 0.238 0.190 Use the integrated rate law to determine the order of the reaction and write the rate law and determine the value of k for this degradation reaction.arrow_forwardFor the gas phase decomposition of nitrogen dioxide at 383 °C2 NO2 -------> 2 NO + O2the following data have been obtained: [NO2], M 0.423 0.178 0.113 7.61x10-2 time, s 0 6.00 12.0 18.0 The average rate of disappearance of NO2 over the time period from t = 6.00 s to t = 12.0 s is ________ M s-1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY