
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
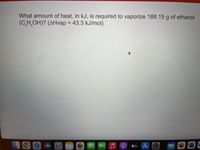
Transcribed Image Text:What amount of heat, in kJ, is required to vaporize 188.15 g of ethanol
(C,H OH)? (AHvap = 43.3 kJ/mol)
étv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How much heat is required to vaporize 35.8 gg of water at 100 ∘C∘C? (ΔHvap(H2O)=40.7kJ/mol)arrow_forwardHow much heat energy (in J) is involved when 55 grams of gaseous water, at 107 °C is cooled to 56° C? (Given: ∆Hvap = +40.65 kJ/mol, CH2O(g) = 1.84 J/g°C, CH2O(l) = 4.184 J/g°C). Watch your sign for q!arrow_forwardWhat quantity of heat is required to convert 49 g of ethanol (C₂H₅OH) at 23.0°C to a vapor at 78.3°C (its boiling point)? (heat capacity of ethanol = 2.46 J/g • C; ∆Hvap = 39.3 kJ/mol)arrow_forward
- Calculate the energy released as heat when 18.7 g of liquid mercury at 25.00 °C is converted to solid mercury at its melting point. heat capacity of Hg(1) Constants for mercury at 1 atm melting point enthalpy of fusion 28.0 J/(mol-K) # 234.32 K 2.29 kJ/mol 9 = MacBook Proarrow_forwardWhat amount of heat (in kJ) is required to convert 15.5 g of an unknown solid (MM = 83.21 g/mol) at -5.00 °C to a liquid at 52.3 °C? (specific heat capacity of solid = 2.39 J/g・°C; specific heat capacity of liquid = 1.58 J/g・°C; ∆Hfus = 3.72 kJ/mol; normal freezing point, Tf = 10.3°C)arrow_forwardA 0.554 g sample of steam at 106.0 °C is condensed into a container with 5.20 g of water at 16.4 °C. What is the final temperature of the water mixture if no heat is lost? The specific heat of water is 4.18, the specific heat of steam is 2.01 g., and AH vap = 40.7 kJ/mol. T₁ = °Carrow_forward
- What amount of heat , in kj, is required to vaporized 190.55 g of ethanol (C2H5OH)?(Hvsp=43.3 KJ/mol)arrow_forwardWhen 95.0 grams of ethanol condenses to liquid, how much energy is lost? H(vap) 38.6 kJ/mol and H(fus) 4.60 kJ/mol are the heats of vaporization and fusion, respectively, for ethanol. Ethanol has a molecular weight of 46.arrow_forwardWhat quantity of heat, in kJ, is required to convert 12.0 g of ethanol (C₂H₂OH) at 23.0°C to a vapor at 78.3°C (its boiling point)? (specific heat capacity of ethanol = 2.46 J/g C; AHvap = 39.3 kJ/mol) ●arrow_forward
- Table B Physical Constants for Water 334 /g Heat of Fusion 2260 J/g Heat of Vaporization 4.18 J/g•K Specific Heat Capacity of H,O(e)arrow_forwardEnter your answer in the provided box. From the data below, calculate the total heat (in J) needed to convert 0.821 mol of gaseous ethanol at 300.0°C and 1 atm to liquid ethanol at 25.0°C and 1 atm: b.p. at 1 atm: 78.5°C Cgas: 1.43 J/g °C AHⓇ : 40.5 kJ/mol vap Cliquid 2.45 J/g.°Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY