
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:101 Chem101
Pt Periodic Table - Ptable
Answered: Using the data in the
cf2o lewis structure - Google Sea X
O X
A app.101edu.co
Question 13 of 34
Submit
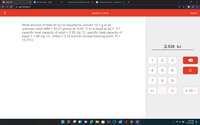
What amount of heat (in kJ) is required to convert 10.1 g of an
unknown solid (MM = 83.21 g/mol) at -5.00 °C to a liquid at 52.3 °C?
(specific heat capacity of solid = 2.39 J/g-°C; specific heat capacity of
liquid = 1.58 J/g•°C; AHfus = 3.72 kJ/mol; normal freezing point, Tf =
10.3°C)
2.539 kJ
1
2
4
C
7
8
9.
+/-
х 100
8:02 PM
12/13/2021
...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Two 20.0 g ice cubes at -17.00C are placed into 470.1 g of water at 25.0 0C. The heat of fusion of ice is 6.008 kJ/mol, the molar heat capacity of water is 75.3 J mol-1 K-1 and the molar heat capacity of ice is 37.7 J mol-1 K-1. Assuming no energy is transferred to or from the surroundings, please calculate the final temperature of the water after all the ice melts. _______ 0Carrow_forwardLiquid butane is used in cigarette lighters. The boiling point of butane at 1 atm pressure is -1.0 °C and its ∆H(vap) is 22.44 kJ/mol. R = 8.314 ×10⁻³ kJ/mol・K. Calculate the pressure (in atm) of the butane in the lighter at 19.5 °C.arrow_forwardEthanol (C₂H₂OH) meits at-114 °C and boils at 78°C. The enthalpy of fusion of ethanol is 5.02 kJ/mol, and its enthalpy of vaporization is 38.56 kJ/mol. The specific heat of solid and liquid ethanol are 0.97 and 2.3 J/(g-K), respectively. Y Part A How much heat is required to convert 30.5 g of ethanol at 28 °C to the vapor phase at 78 "C? Express your answer in kilojoules to three significant figures. 195] ΑΣΦ Submit Part 8 Request Answer Submit ? How much heat is required to convert 30.5 g of ethanol at-160 C to the vapor phase at 78 "C7 Express your answer in kilojoules to two significant figures. VA24 Request Answer kJ kJarrow_forward
- 13) Ethanol melts at -114 C and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kJ/mol, and its enthalpy of evaporation is 38.56 kJ/mol. The specific heats of solid and liquid ethanol are 0.97 and 2.3 J/gK, respectively. How much heat is required to convert 42.0 g of ethanol at 35 °C to the vapor phase at 78 °C?arrow_forwardSpecific heat of H,O(s) 2.087 J/(g · °C) Specific heat of H,O(1) |4.184 J/ (g · °C) Heat of fusion for H,O A total of 865 cal of heat is added to 5.00 g of ice at -20.0 °C. 333.6 J/g What is the final temperature of the water? °C Tinalarrow_forwardWhat is the vapor pressure of ethanol in (mmHg) at 62.5°C if it's vapor pressure is 400.0 mmHq at 63,5°c? | AH vap= =99.3 KJ/mol; R= 8.314 S/kimoi>arrow_forward
- Sulfur dioxide is produced in enormous amounts for sulfuric acid production. It melts at −73.0 ° C and boils at −10.0 ° C. Its Δ H o fus is 8.619 kJ/mol, and its Δ H o vap is 25.73 kJ/mol. The specific heat capacities of the liquid and gas are 0.995 J/g · K and 0.622 J/g · K, respectively. How much heat is required to convert 7.750 kg of solid SO2 at the melting point to a gas at 60.0 ° C?arrow_forwardWhen 5.00 g of isopropyl alcohol ((CH3),CHOH; molar mass = point of 82.6°C, it absorbs 3.66 kJ of heat. What is the enthalpy of vaporization of isopropyl alcohol in kJ/mol? 60.09 g/mol) evaporates at its boilingarrow_forwardHow much heat energy is required to convert 69.8 g69.8 g of solid iron at 24 ∘C24 ∘C to liquid iron at 1538 ∘C?1538 ∘C? The molar heat of fusion of iron is 13.8 kJ/mol. Iron has a normal melting point of 1538 ∘C.1538 ∘C. The specific heat capacity of solid iron is 0.449 J/(g·∘C).arrow_forward
- calculate the total amount of heat needed to convert 22.50 g of solid carbon tetrachloride, CCl4 at a temperature of -35 C to vapor form at 120 C, given the following values for CCl4 : melting point= -22.9 C; boiling point = 76.2C; change in H fusion = 2.69 kj/mol ; change in H vapor =29.8 kj/molarrow_forwardWhat quantity of heat, in kJ, is required to convert 12.0 g of ethanol (C₂H₂OH) at 23.0°C to a vapor at 78.3°C (its boiling point)? (specific heat capacity of ethanol = 2.46 J/g C; AHvap = 39.3 kJ/mol) ●arrow_forwardWhat quanity of energy does it take to convert 0.451 kg ice at -20.0 degrees celcius to steam at 250.0 degrees celcius? Specific heat capacities: Ice, 2.03J/g degrees celcius; liquid, 4.18 J/g degrees celcius; steam, 2.02 J/g degrees celcius. delta H vap = 40.7kJ/mol; deltaHfus = 6.02kJ/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY