Concept explainers
Question
Weather radar systems emit radio waves in pulses. For a typical system the frequency used is 3.00 GHz (1 GHz = 109 Hz) and the minimum energy uncertainty of the radio photons is 5.50 * 10-29 J. Find (a) the time duration of each pulse, (b) the length of each pulse, (c) the energy of an average photon, and (d) the minimum frequency uncertainty.
Expert Solution
arrow_forward
Step 1
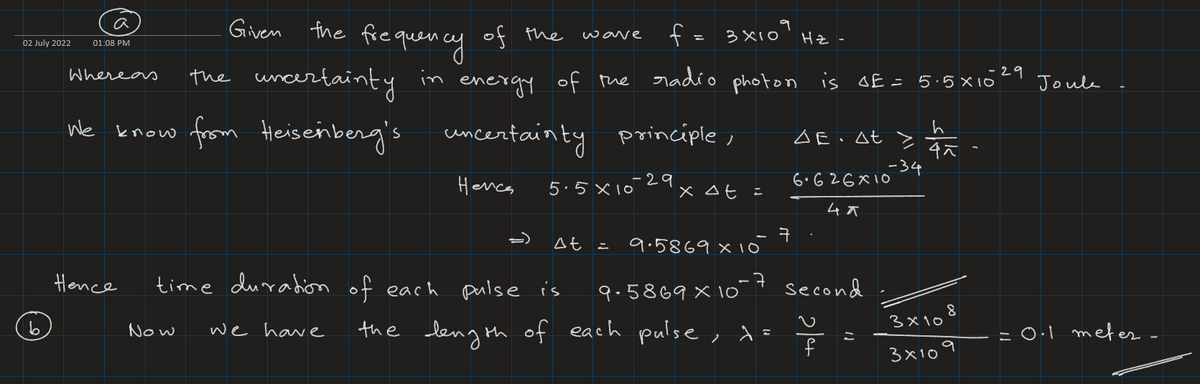
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, advanced-physics and related others by exploring similar questions and additional content below.Similar questions
- Lasers can be designed to emit pulses of light less than 30 microns wide in their direction of motion. The momentum of a photon is given by p = h/λ. Estimate the uncertainty in the wavelength of a photon in the pulse, assuming a nominal wavelength of 800nm.arrow_forwardWhat is the kinetic energy of each electron in a beam of electrons if the beam produces a diffraction pattern of a crystal which is similar to that of a beam of 1.00 eV neutrons? (knowing that electron mass is 9.11*10^-31 kg and neutron mass is 1.67*10^-26 kg). What are the specific equations that are being used in this problem?arrow_forwardIn designing an experiment, you want a beam of photons and a beam of electrons with thesame wavelength of 0.281 nm, equal to the separation of the Na and Cl ions in a crystal ofNaCl. Find the energy of the photons and the kinetic energy of the electrons in electrovolts(eV).arrow_forward
- The velocity of an electron is known to be 1.000×105 m/s, with an uncertainty of Av = 1.00×102 m/s. (a) What is the minimum uncertainty in the electron's position, Av, in meters? (b) How does this compare to the de Broglie wavelength of the electron? (c) One of your professors (m = 75.0 kg) is pacing at the front of the classroom, and you measure their velocity to an uncertainty of Av = 0.100 m/s. What is the minimum uncertainty in a measurement of their position? (d) How does this compare to the height of your professor?arrow_forwardAn object is moving along a straight line, and the uncertainty in its position is 2.20 m. (a) Find the minimum uncertainty in the momentum of the object. Find the minimum uncertainty in the object's velocity, assuming that the object is (b) a golf ball (mass = 0.0450 kg) and (c) an electron. (a) Number (b) Number (c) Number Units Units Unitsarrow_forwardAn atom is in an excited state for 4.00 us before moving back to the ground state. Find the approximate uncertainty in energy of the photon in units of 10¹¹ eV. (A) 8.23 (B) 3.78 (C) 4.97 (D) 5.49 (E) 6.17arrow_forward
arrow_back_ios
arrow_forward_ios