
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:The wall of a spherical steel tank k, = 17
m. K
thick contains hot oil at 100°C. On the outside of the tank 10mm of spray
W
urethane insulation k, = 0.026
m. K
of 1m in diameter and 100 mm
h = 16.
is added to stop the oil from cooling. If the
outside temperature is 25°C,
W
m².K
needs to be added to keep the tank at 100°C for pumping reasons? What are the
overall heat transfer coefficients (Uo and Ui)?
and h = 5
(₁
m². K
how much heat
Expert Solution
arrow_forward
Heat transfer rate
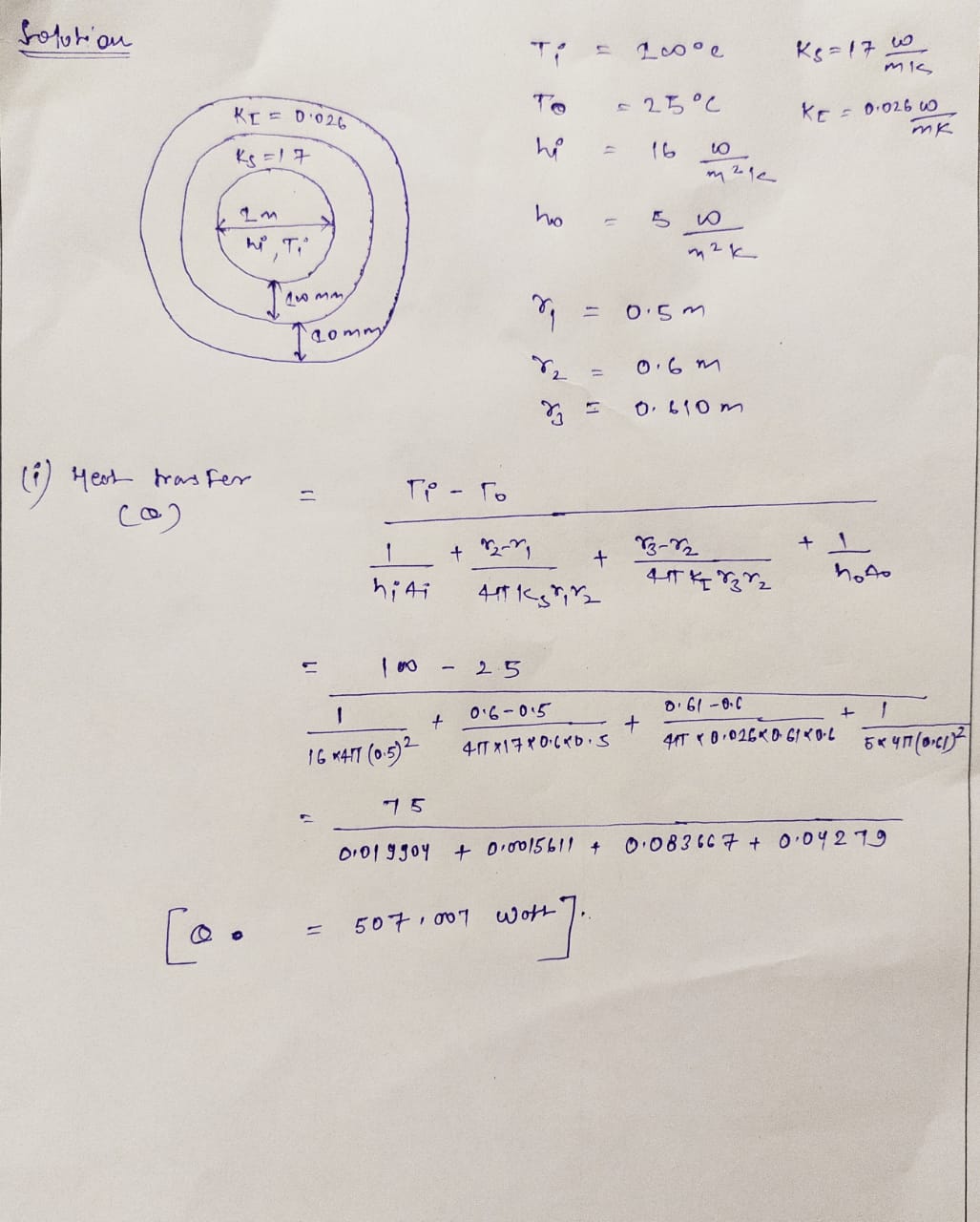
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- A glass window of width W = 1 m and height H = 2 m is 5 mm thick and has a thermal conductivity of k 1.4 W/m K. If the inner and outer surface temperatures of the glass are 15°C and -20°C, respectively, on a cold winter day, what is the rate of heat loss through the glass? To reduce heat loss through windows, it is cus- tomary to use a double pane construction in which adjoining panes are separated by an air space. If the spacing is 10 mm and the glass surfaces in contact with the air have temperatures of 10°C and -15°C, what is the rate of heat loss from a 1 m x 2 m window? The thermal conductivity of air is k, = 0.024 W/m.K.arrow_forwardA metallic sphere of radius R is heated in an oven to a temperature of 600 ° F throughout, and then removed from the oven and allowed to cool in ambient air to T∞ = 75 ° F, by convection and radiation (see attached figure). The thermal conductivity of the ball material is known to vary linearly with temperature. Assuming that the ball is uniformly cooled starting from the entire outer surface, obtain the differential equation that describes the change in temperature in the sphere during cooling. Note: In the image, the translation would be: metallic spherearrow_forwardHollow Sphere Saturated steam at 267°F is flowing inside a hollow sphere with an ID of 0.824 in. and an OD of 1.05 in. The pipe is insulated with 1.5 in. of insulation on the outside. The convective heat transfer coefficient inside and outside the pipe is hi = 1000 Btu/hr/ft2/°F and ho = 2 Btu/hr/ft2/°F, respectively. The mean thermal conductivity of the metal is 45 W/m/K or 26 Btu/hr/ft/°F, while that of the insulation material is 0.064 W/m/K or 0.037 Btu/hr/ft/°F. a. Calculate the heat loss for 1 ft of pipe using resistances if the surrounding air is at 80°F. b. Calculate the temperature profile developed for this system. c. Calculate the inside and outside overall heat transfer coefficients.arrow_forward
- Steel pipe (outer diameter 100 mm) is covered with two layers of insulation. The inner layer, 40 mm thick, has a thermal conductivity of 0.07 W / (m K). The outer layer, 20 mm thick, has a thermal conductivity of 0.15 W / (m K). Pipes are used to deliver steam with a pressure of 700 kPa. The temperature on the outer insulation surface is 24 ° C. If the pipe is 10 m long, determine the following: (assuming that the conduction heat transfer resistance of the steel pipe and the vapor convection resistance are negligible). a. Heat loss per hour. = AnswerkJ / hour. b. Temperature between insulation layers. = Answer° C.arrow_forwardA large slab of aluminum has a thickness of 10 cm and is initially uniform in temperatureat 400◦C. It is then suddenly exposed to a convection environment at 90◦Cwith h=1400 W/m2 · ◦C. How long does it take the center to cool to 180◦C?arrow_forwardQ: water at 200² flows over 90.3m square Plate at velocity of 6 m/s at constant Plate is maintained Temperature at 556° calclate the heat by the plate. heat Transferarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The