
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
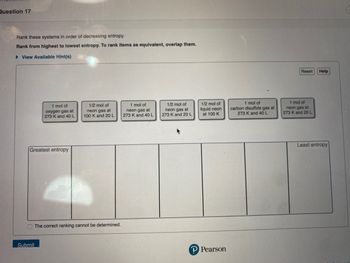
Transcribed Image Text:Question 17
Rank these systems in order of decreasing entropy.
Rank from highest to lowest entropy. To rank items as equivalent, overlap them.
► View Available Hint(s)
1 mol of
oxygen gas at
273 K and 40 L
Greatest entropy
Submit
1/2 mol of
neon gas at
100 K and 20 L
The correct ranking cannot be determined.
1 mol of
neon gas at
273 K and 40 L
1/2 mol of
neon gas at
273 K and 20 L
1/2 mol of
liquid neon
at 100 K
P Pearson
1 mol of
carbon disulfide gas at
273 K and 40 L
Reset
1 mol of
neon gas at
273 K and 20 L
Help
Least entropy
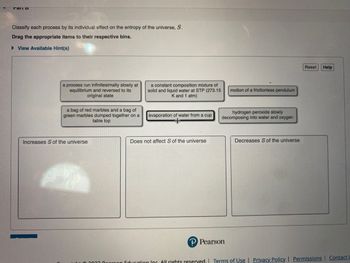
Transcribed Image Text:Mail D
Classify each process by its individual effect on the entropy of the universe, S.
Drag the appropriate items to their respective bins.
►View Available Hint(s)
a process run infinitesimally slowly at
equilibrium and reversed to its
original state
a bag of red marbles and a bag of
green marbles dumped together on a
table top
Increases S of the universe
a constant composition mixture of
solid and liquid water at STP (273.15
K and 1 atm)
evaporation of water from a cup
+
Does not affect S of the universe
motion of a frictionless pendulum
hydrogen peroxide slowly
decomposing into water and oxygen
P Pearson
Decreases S of the universe
Reset
Help
con Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions Contact L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict whether AS for each reaction would be greater than zero, less than zero, or too close to zero to decide. H₂(g) + Cl₂ (g) → 2HCl(g) COC12 (g) → CO(g) + Cl₂(g) CO2(g)+H2(g) → CO(g) +H,O(g) 2CO2 (g) + 5H2(g) → C₂H₂(g) + 4H₂O(g) 2H₂O(1)→ 2H₂(g) + O₂(g) Show Hint Clear All AS > 0 AS <0 too close to decide Previous Email Instructor Next Save andarrow_forwardC2H5OH(1) has a higher entropy value than C2H5OH(g) A true b falsearrow_forwardH+(aq) + OH-(aq) - - -> H2O (l) Use the table below to determine net ∆H for this reaction.arrow_forward
- 7arrow_forward5. Which of the following do you expect to have the largest molar entropy at 25 °C? a) H₂O (liq) b) H₂O (s) c) O₂ (g) d) CCl4 (g) e) same for all the casesarrow_forwardFor each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S , or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. System Change S A few moles of carbon dioxide (CO2) gas. The carbon dioxide is heated from 9.0 °C to 36.0 °C and also expands from a volume of 6.0 L to a volume of 14.0 L. <S0 =S0 >S0 not enough information A few moles of helium (He) gas. The helium expands from a volume of 9.0 L to a volume of 15.0 L while the temperature is held constant at 20.0 °C. <S0 =S0 >S0 not enough information A few grams of liquid ammonia (NH3). The ammonia is heated from -1.0 °C to 2.0 °C. <S0…arrow_forward
- D Try Again Your answer is incorrect. Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A • reaction A: Your answer is incorrect. • reaction C: Your answer is incorrect. B с observations The reverse of this reaction is always spontaneous. This reaction is exothermic and proceeds slower at temperatures below -26. °C. This reaction is always spontaneous, but proceeds faster at temperatures above 139. °C. AH is AS is conclusions AH is As is AH is AS is X unknown negative ŷ negative ↑ unknown î unknown ↑ positive î Sarrow_forwardEnter your answer in the provided box. Calculate the standard entropy change for the following reaction at 25°C. S(rhombic) + O:(g) - so:g) K'mol S(rhombie) 31.S8 205.0 248.5 J/K-molarrow_forwardDetermine the entropy change in each of the following processes: 4a. One mole of an ideal gas is allowed to expand from a volume of 1.50 L to 15.0 L at 298 K. 4b. 10 grams of methanol vapor, initially at its normal boiling temperature of 337 K, is allowed to condense and cool to a liquid at a final temperature of 298 K.arrow_forward
- Number 4 part barrow_forwardFor each pair listed below, which choice has the substance with the highest entropy listed first? O2(g), air (a mixture of O2 and N2 gases) CH4(g), C3H3(g) NO(g), NO2(1) Li(s) @ 25 K, Li(s) @ 25 °Carrow_forwardWhich of the following represents a process that is always spontaneous? ASsys: negative ASsurr: negative ASsys: positive ASsurr: positive ASsys: positive ASsurr: negative ASsys: negative ASsurr: positivearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY