
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
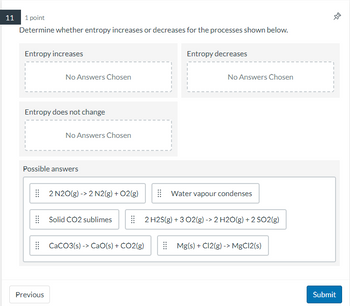
Transcribed Image Text:11 1 point
Determine whether entropy increases or decreases for the processes shown below.
Entropy increases
No Answers Chosen
Entropy does not change
Previous
No Answers Chosen
Possible answers
2 N2O(g) -> 2 N2(g) + O2(g)
Solid CO2 sublimes
CaCO3(s)-> CaO(s) + CO2(g)
Entropy decreases
No Answers Chosen
Water vapour condenses
2 H2S(g) + 3 O2(g) -> 2 H2O(g) + 2 SO2(g)
Mg(s) + C12(g) -> MgCl2(s)
42
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Ammonia can be made from the reaction of nitrogen and hydrogen: N2 (g) + 3 H2 (g) ⟺ 2 NH3 (g) For this reaction, K = 1.6 x 102 and ΔH = -91.8 kcal/mole at 25oC. Is the reaction endothermic or exothermic? Briefly explain. Is there in increase in entropy, a decrease in entropy, or no change in entropy? Briefly explain. At 25oC, is this reaction product favored or reactant favored? Briefly explain. Do you expect this reaction to be spontaneous at all temperatures? Briefly explain.arrow_forwardFor each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S , or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. System Change S A few moles of carbon dioxide (CO2) gas. The carbon dioxide is heated from 9.0 °C to 36.0 °C and also expands from a volume of 6.0 L to a volume of 14.0 L. <S0 =S0 >S0 not enough information A few moles of helium (He) gas. The helium expands from a volume of 9.0 L to a volume of 15.0 L while the temperature is held constant at 20.0 °C. <S0 =S0 >S0 not enough information A few grams of liquid ammonia (NH3). The ammonia is heated from -1.0 °C to 2.0 °C. <S0…arrow_forward1:44 1 LTE 4 1 Search Question 20.b of 23 Show Answer Consider the reaction Cl2(g) + Br2(g) → 2 BrCI(g) at 25 °C. Which of the following best explains why the change in entropy is so small? A) All of the components in the chemical equation are gases. B) There are the same moles of gas on both sides of the reaction. C) The temperature of the reaction does not change. Tap here or pull up for additional resourcesarrow_forward
- Which reaction below has a positive change in entropy? O 2 N20 (g) --> 2 N2 (g) O2 (e) O N2 (g) + 3 H2 (g) -- 2 NH3 (g) O 2 KCIO3 (s) --> 2 KCI (s) + 3 O2 (g) O 2 H2 (e) C2H2 (g)--> C2H, (g).arrow_forwardIp acellus_engine.html?ClassID=D1653724337# Calculate the entropy of the phase change of uranium hexafluoride from gas to solid. UF6(g) → UF6(s) Substance S° (J/mol-K) UF (s) UF (g) ASiep = [ ? ] J/mol - K 228 380 Enter either a + or - sign AND the magnitude in your answer. Enterarrow_forward1)spontaneous or non spontaneous 2) the change in Gibbs free energy is negative or the change in Gibbs free energy is positive 3)positive or negativearrow_forward
- question will save this response. Question 17 Using the entropy values in the table below, what is the change in standard entropy, ASº, for the following reaction? 2 CH3OH (1)+3 02 (g)→2 CO2 (g) + 4 H2O (g) Substance S° (J/mol K) CH3OH (1) CH3OH (g) 02 (g) CO2 (g) H2O (g) 127 238 205 214 189 H20 (1) 240 J/K 70 93 J/K 315 J/K -161 J/K O-383 J/K A Moving to another question will save this response. MacBook Air 30 Qa F4 F5 F3 F1 F2 % %24arrow_forwardgiven rgetable of standard molar entropy values of each substance in the reaction below, what is the standard change in entropy, delta s, for the following reaction? 2CH3OH(g)+3OH2- 2CO2(g)+4H2O(g) Substance= CH3OH(g) S(J/mol•K) 240 Substance=O2(g) S(J/mol•K) 205 Substance=CO2(g) S(J/mol•K) 214 Substance=H2O(g) S(J/mol•k) 189 a. -352 J/K b. -1302 J/K c. 315 J/K d. 89 J/K E. 1830 J/Karrow_forwardFor each system listed in the first column of the table below, decide (if possible) whether the change described in the second column will increase the entropy S of the system, decrease S, or leave S unchanged. If you don't have enough information to decide, check the "not enough information" button in the last column. System Change AS O AS 0 not enough information O AS 0 not enough O information O AS 0 not enough O information Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privacy Center | O Oarrow_forward
- Please don't provide handwriting solutionarrow_forwardItem 3 Constants | Periodic Table Part A Which one of the following processes produces a decrease in the entropy of the system? O mixing of two gases into one container O freezing of Fe(1) into Fe(s) O evaporation of liquid ethanol into gaseous ethanol O melting solid lead into liquid lead O dissolution of KCl(s) in water Submit Request Answerarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY