
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The following results were obtained for Tray n from a rate-basedcalculation of a ternary distillation at 14.7 psia, involving acetone (1), methanol (2), and water (3) in a 5.5-ft-diameter column using sieve trays with a 2-inch-high weir. Vapor and liquid phases are assumed to be completely mixed.The computed products of the gas-phase, binary mass-transfer coefficients
and interfacial area, using the Chan–Fair correlation of §6.6,are as follows in lbmol/:
k12 =k21=1, 955; k13 =k31 =2,407; k23=k32 = 2,797
(a) Compute the molar diffusion rates. (b) Compute the masstransfer
rates. (c) Calculate the Murphree vapor-tray efficiencies.
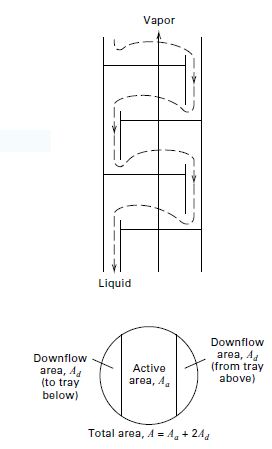
Transcribed Image Text:Vapor
Liquid
Downflow
area, A4
(from tray
above)
Downflow
area, A
(to tray
below)
Active
area, A.
Total area, A= A, + 244
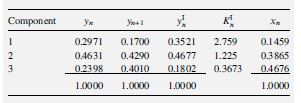
Transcribed Image Text:Ул
K,
2.759
1.225
Component
0.2971
0.4631
0.3521
0.1700
0.1459
0.4677
0.1802
1.0000
0.4290
0.3865
0.2398
1.0000
0.4010
1.0000
0.3673
04676
1.0000
Expert Solution
arrow_forward
Step 1

arrow_forward
Step 2

Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A one-inch thick steak initially at room temperature is placed inside an oven maintained at 250 o C. Estimate how long it will take for its internal temperature to reach 70 o C. What will be the surface temperature at that time if we assume no steam generation? Include radiation as well as convection (h ~ 3 W/m²/K) in your analysis.arrow_forwardI need this ASAParrow_forward1. Describe this diagram in terms of what information it gives you and how you can use this to determine a heat treatment for steel (10). 2. Describe a simple continuous cooling heat treatment to a) Bainite to pearlite 3. Describe the difference between the microstructure of bainite and tempered pearlitearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The