
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![**Using the values in Table 6.2, give ΔH for each reaction, and classify the reaction as endothermic or exothermic.**
**a.**
\[ \text{H-Br:} \rightarrow \text{H} \cdot + \cdot \text{Br:} \]
\[ \Delta H = \_\_\_\_ \text{kcal/mol} \]
(Select: Endothermic/Exothermic)
---
**b.**
\[ \text{H} \cdot + \cdot \text{Cl:} \rightarrow \text{H-Cl} \]
\[ \Delta H = \_\_\_\_ \text{kcal/mol} \]
(Select: Endothermic/Exothermic)
---
**c.**
\[ \text{H-OH} \rightarrow \text{H} \cdot + \cdot \text{OH} \]
\[ \Delta H = \_\_\_\_ \text{kcal/mol} \]
(Select: Endothermic/Exothermic)
---
**Explanation:**
This exercise involves calculating the enthalpy change (ΔH) for each of the given chemical reactions using data from Table 6.2 (not provided). You will then classify each reaction as either endothermic (absorbing heat) or exothermic (releasing heat).
Each part (a, b, c) represents a chemical reaction with spaces to fill in the ΔH value and to select whether the reaction is endothermic or exothermic. The dot (•) represents an unpaired electron, indicating the species are radicals.](https://content.bartleby.com/qna-images/question/2ecf5b80-2518-4246-9cf0-28fbc9ab655e/8cd3c29c-29d7-4975-8b7c-e70492d2a476/gmgc7ci_thumbnail.jpeg)
Transcribed Image Text:**Using the values in Table 6.2, give ΔH for each reaction, and classify the reaction as endothermic or exothermic.**
**a.**
\[ \text{H-Br:} \rightarrow \text{H} \cdot + \cdot \text{Br:} \]
\[ \Delta H = \_\_\_\_ \text{kcal/mol} \]
(Select: Endothermic/Exothermic)
---
**b.**
\[ \text{H} \cdot + \cdot \text{Cl:} \rightarrow \text{H-Cl} \]
\[ \Delta H = \_\_\_\_ \text{kcal/mol} \]
(Select: Endothermic/Exothermic)
---
**c.**
\[ \text{H-OH} \rightarrow \text{H} \cdot + \cdot \text{OH} \]
\[ \Delta H = \_\_\_\_ \text{kcal/mol} \]
(Select: Endothermic/Exothermic)
---
**Explanation:**
This exercise involves calculating the enthalpy change (ΔH) for each of the given chemical reactions using data from Table 6.2 (not provided). You will then classify each reaction as either endothermic (absorbing heat) or exothermic (releasing heat).
Each part (a, b, c) represents a chemical reaction with spaces to fill in the ΔH value and to select whether the reaction is endothermic or exothermic. The dot (•) represents an unpaired electron, indicating the species are radicals.
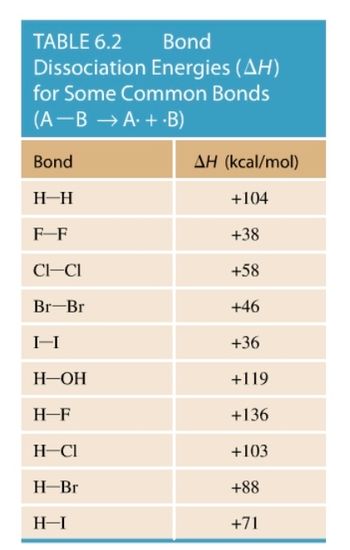
Transcribed Image Text:**Table 6.2: Bond Dissociation Energies (ΔH) for Some Common Bonds**
This table presents the bond dissociation energies, which are measures of the strength of chemical bonds. The energy is expressed in kilocalories per mole (kcal/mol) and denotes the energy required to break the bond between atoms A and B, resulting in free radicals A· and ·B.
- **H—H**: +104 kcal/mol
- **F—F**: +38 kcal/mol
- **Cl—Cl**: +58 kcal/mol
- **Br—Br**: +46 kcal/mol
- **I—I**: +36 kcal/mol
- **H—OH**: +119 kcal/mol
- **H—F**: +136 kcal/mol
- **H—Cl**: +103 kcal/mol
- **H—Br**: +88 kcal/mol
- **H—I**: +71 kcal/mol
This information is crucial for understanding the stability and reactivity of molecules in various chemical reactions. Higher ΔH values signify stronger bonds that require more energy to break.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Dd.101.arrow_forwardA salt has a solubility in water of 2.5 g/L at 25 °C Suppose you mixed 10.0g of this salt with 3.0 L of water in a beaker and allowed the salt to dissolve. The temperature of the solution was kept at 25 °C. Would there be any solid left in the beaker after the salt has dissolved? Select one: O Yes, there would be some solid would be left in the beaker. O No, there would not be any solid left in the beaker. Not enough information is provided to answer this questionarrow_forwardA chemical reaction doesn't necessarily involve electrons. °F Mostly sunny F1 O ! 1 Q A N True F2 2 W S F3 Alt -8+ X #M 3 E F4 D ta + $ 4 C F5 R 2 F % 5 F6 V T G F7 6 Y B F8 & 7 H U N F9 *00 8 J F10 ( 9 M O False K GO F11 O @ ) 0 < F12 L P Altarrow_forward
- PbCrO4 is a common pigment called chrome yellow (or school bus yellow). It has a solubility of 1.71 x 10-4 g/L. The solubility product of PbCrO4, Ksp = ________. Enter the result in scientific notation to 1 decimal. e.g. enter 5.6x10-5 as 5.6E-5.arrow_forwardClassify each chemical reaction: CuSO₂ (aq) + ZnCrO₂ (aq) → ZnSO₂ (aq) + CuCrO₂ (s) reaction H,CO,(aq) → H,O(1) + CO,(g) HI (aq) + NaOH(aq) Nal (aq) + H₂O(1) HBr(aq) + NaOH(aq) → NaBr(aq) +H,O(l) Explanation Check type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition X precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base S © 2023 McGraw Hill LLC. All Rights Rarrow_forwardessentially buming, fuels react with oxygen to redease energy. Balance the reactions: NazS+ Zn(NO,) → Na(NO3) + Zn2S | Li+ N2 LijN LizN KCIO→ KCI +_02 CH4 + 02- НО + CO2arrow_forward
- 100 90 80 NANO, 70 60 CaCl 50 Pb(NO.)2 40 NaCi KCI 30 20 KCIO, 10 Ce,(SO O 10 20 30 40 50 60 70 80 90 100 Temperature ("C) How many grams are needed to create a saturated solution of Pb(NO3)2 at 30°C if you have 20 grams of Pb(NO3), already dissolved in a solution? Solubility (g of salt in 100 g H,O) SONYarrow_forwardQuestion 2 Use the following information to answer the question. A technician prepares 500 mL of a 0.100 mol/L solution of acetic (ethanoic) acid by diluting a concentrated solution of acetic acid with water. The equilibrium equation that represents the reaction that occurs when acetic acid and water react is shown below. CH,COOH(aq) + H₂O(1) CH,CO0¹ (aq) + H₂O*(aq) a. For the prepared acetic acid solution, calculate the concentration of H3O*(aq) and the pH. CH₂COOH + H₂O. → CH₂COO H30 V= 0.5L C0.100m0111 n=cv-> (0-100) (0.5) => 0.05 mol = =0.0smol. neo 05 nul Show Transcribed Text Use the following additional information to answer the next part of the question. A technician uses the 500 mL of 0.100 mol/L acetic acid to prepare an acetic acid-sodium acetate buffer solution. She does this by adding 0.100 mol/L sodium acetate to the 0.100 mol/L acetic acid until the required pH is reached. b. Identify the shift in equilibrium that will occur when sodium acetate is added to the…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY