
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
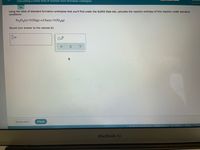
Transcribed Image Text:Calculating a molar heat of reaction from formation enthalpies
anbɔec
Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard
conditions:
Fe,03(s)+3 CO(g)→2 Fe(5)+3 CO,(g)
Round your answer to the nearest kJ.
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Uss Privacy Acces
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: 2C2H6 (g) + 7O2 (g) → 4CO2 (g) + 6H2O (l)Round your answer to the nearest kJ .arrow_forwardUsing the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: 2 HNO₂(g) + O₂(g) →2 HNO3(g) Round your answer to the nearest kJ. ៣ kJ x10 Xarrow_forwardUsing the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: 2CH3OH (l) + 3O2 (g) → 2CO2 (g) + 4H2O (l)Round your answer to the nearest kJ .arrow_forward
- You mix 125 mL of 0.250 M CSOH with 50.0 mL of 0.625 M HF in a coffee-cup calorimeter, and the temperature of both solutions rises from 21.70 °C before mixing to 24.59 °C after the reaction. CsOH(aq) + HF (aq) → CsF(aq) + H₂O(l) What is the enthalpy of reaction per mole of CSOH? Assume the densities of the solutions are all 1.00 g/mL, and the specific heat capacities of the solutions are 4.2 J/g. K. Enthalpy of reaction = kJ/molarrow_forwardUsing the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: 2NaOH (s) + CaCl2 (s) → CaOH2 (s) + 2NaCl (s)Round your answer to the nearest kJ .arrow_forwardA chemist measures the enthalpy change AH during the following reaction: 2 KCl(s) + 302(g) →2 KClO3(s) AH=78. kJ Use this information to complete the table below. Round each of your answers to the nearest kJ. reaction ΔΗ ㅁ x10 2K C103 (s) → 2KC1(s) + 302(g) KCIO, (s)-KCI(s) + 0,(s) 3 KCI(s)+0, (g)KCIO, (s) kJ kJ 1 5arrow_forward
- Use the References to access important values if needed for this question. A scientist measures the standard enthalpy change for the following reaction to be -103.2 kJ: 4HCl(g) + O2(g) → 2H₂O(g) + 2Cl₂ (g) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of HCl(g) is I kJ/mol red Species AH (kJ/mol) H₂O(g) -241.8 Cl₂(g) 0.0 0₂ (9) 0.0arrow_forwardA chemist measures the enthalpy change AH during the following reaction: H₂O(1)→ H₂O(g) ΔΗ = 44. kJ Use this information to complete the table below. Round each of your answers to the nearest kJ/mol. reaction 3H₂O(g) → 3H₂O (1) 4H₂O(1)→ 4H₂O(g) H₂O(g) → H₂O (1) AH kJ x10 X Śarrow_forwardFind the enthalpy change for this reaction using the data below. Clearly show how you got the answer. 2 Al(s) + 3 Cl2 (g) → 2 AlCl3 (s) Data: (a) 2 Al(s) + 6 HCl(aq) → 2 AlCl3 (aq) + 3 H2 (g) H = −1049 kJ (b) AlCl3 (s) → AlCl3 (aq) H = −323 kJ (c) H2 (g) + Cl2 (g) → 2 HCl(g) H = −185 kJ (d) HCl(aq) → HCl(g) H = +74.8 kJarrow_forward
- A scientist measures the standard enthalpy change for the following reaction to be -912.5 kJ:2NH3(g) + 3 N2O(g)4N2(g) + 3 H2O(g)Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of H2O(g) is kJ/mol.arrow_forward(1)Consider the reaction: 2A (g) + 3 B (g) → 2 C (g) ΔHrxn = +254.3 kJ What will be the enthalpy change (in kJ) if 0.471 mol B reacts in excess A? (2)Consider the reaction: C (s) + O2 (g) → CO2 (g) ΔHrxn = -393.5 kJ What mass of carbon (in g) must be reacted via this mechanism to release 581.2 kJ of heat?arrow_forwardGiven the following reaction: 2NO (g) + O2 (g) → 2NO2 (g) ΔrH0 = - 114.1 kJ/mol Calculate the enthalpy change when 1.25 g of NO gas is converted completely converted to NO2 gas. Enter your answer to 2 decimal places.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY