
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
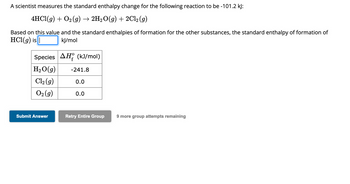
Transcribed Image Text:A scientist measures the standard enthalpy change for the following reaction to be -101.2 kJ:
4HCl(g) + O₂ (g) → 2H₂O(g) + 2Cl2 (g)
Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of
HCl(g) is |
kJ/mol
Species AH (kJ/mol)
H₂O(g)
Cl₂ (g)
O₂(g)
Submit Answer
-241.8
0.0
0.0
Retry Entire Group 9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature of the cooling water as it leaves the hot engine of an automobile is 240 F. After it passes through the radiator it has a temperature of 175 F. Calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a specific heat of 4.184 J/g oC.arrow_forwardA 0.470-g sample of magnesium reacts with 200 g dilute HCl in a coffee-cup calorimeter to form MgCl2(aq) and H2(g). The temperature increases by 10.9 C as the magnesium reacts. Assume that the mixture has the same specific heat as water and a mass of 200 g. (a) Calculate the enthalpy change for the reaction. Is the process exothermic or endothermic? (b) Write the chemical equation and evaluate H.arrow_forwardIn a calorimetric experiment, 6.48 g of lithium hydroxide, LiOH, was dissolved in water. The temperature of the calorimeter rose from 25.00C to 36.66C. What is H for the solution process? LiOH(s)Li(aq)+OH(aq) The heat capacity of the calorimeter and its contents is 547 J/C.arrow_forward
- In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: Fe2O3(s)+3CO(g)2Fe(s)+3CO2(g)H=24.8kJ The enthalpy change for the combustion of carbon monoxide is 2CO(g)+O2(g)2CO2(g)H=566kJ Use this information to calculate the enthalpy change for the equation 4Fe(s)+3O2(g)2Fe2O3(s)H=?arrow_forwardA 50-mL solution of a dilute AgNO3 solution is added to 100 mL of a base solution in a coffee-cup calorimeter. As Ag2O(s) precipitates, the temperature of the solution increases from 23.78 C to 25.19 C. Assuming that the mixture has the same specific heat as water and a mass of 150 g, calculate the heat q. Is the precipitation reaction exothermic or endothermic?arrow_forwardAnother reaction that is used to propel rockets is N2O4(l)+2N2H4(l)3N2(g)+4H2O(g) This reaction has the advantage that neither product is toxic, so no dangerous pollution is released. When the reaction consumes 10.0 g liquid N2O4, it releases 124 kJ of heat. (a) Is the sign of the enthalpy change positive or negative? (b) What is the value of H for the chemical equation if it is understood to be written in molar quantities?arrow_forward
- Given the following (hypothetical) thermochemical equations: A+B2C;H=447kJA+3D2E;H=484kJ2D+B2F;H=429kJ Calculate H, in kJ, for the equation 4E+5B4C+6Farrow_forwardThe combustion of 1.00 mol liquid methyl alcohol (CH3OH) in excess oxygen is exothermic, giving 727 kJ of heat. (a) Write the thermochemical equation for this reaction. (b) Calculate the enthalpy change that accompanies the burning 10.0 g methanol. (c) Compare this with the amount of heat produced by 10.0 g octane, C8H18, a component of gasoline (see Exercise 5.41).arrow_forwardA 10.00-g sample of acetic acid, HC2H3O2, was burned in a bomb calorimeter in an excess of oxygen. HC2H3O2(l)+2O2(g)2CO2(g)+2H2O(l) The temperature of the calorimeter rose from 25.00C to 35.84C. If the heat capacity of the calorimeter and its contents is 13.43 kJ/C, what is the enthalpy change for the reaction?arrow_forward
- A 110.-g sample of copper (specific heat capacity = 0.20 J/C g) is heated to 82.4C and then placed in a container of water at 22.3C. The final temperature of the water and copper is 24.9C. What is the mass of the water in the container, assuming that all the heat lost by the copper is gained by the water?arrow_forwardWhen solid iron burns in oxygen gas (at constant pressure) to produce Fe2O3(s), 1651 kJ of heat is released for every 4 mol of iron burned. How much heat is released when 10.3 g Fe2O3(s) is produced (at constant pressure)? What additional information would you need to calculate the heat released to produce this much Fe2O3(s) if you burned iron in ozone gas, O3(g), instead of O2(g)?arrow_forwardQuinone is an important type of molecule that is involved in photosynthesis. The transport of electrons mediated by quinone in certain enzymes allows plants to take water, carbondioxide, and the energy of sunlight to create glucose. A 0.1964-g sample of quinone (C6H4O2) is burned in a bomb calorimeter with a heat capacity of 1.56 kJ/C. The temperature of the calorimeter increases by 3.2C. Calculate the energy of combustion of quinone per gram and per mole.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning