
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Cc.58.
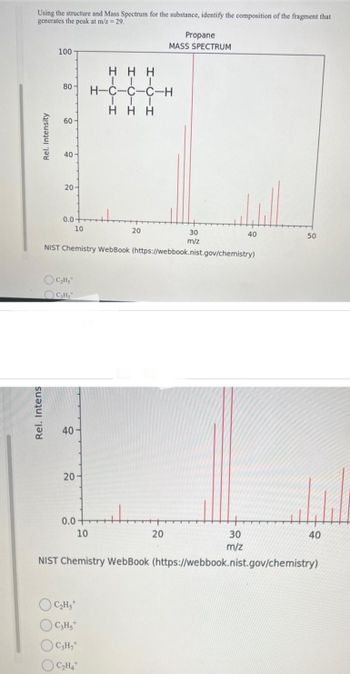
Transcribed Image Text:Using the structure and Mass Spectrum for the substance, identify the composition of the fragment that
generates the peak at m/z = 29.
Rel. Intens
Rel. Intensity
100
80
60-
40-
20-
0.0
C₂H5
C₂H₂
30
m/z
NIST Chemistry WebBook (https://webbook.nist.gov/chemistry)
40
10
20-
0.0
10
HHH
|||
H-C-C-C-H
III
Η Η Η
H
OC₂Hst
C3Hs
OC₂H₂+
C₂H4
20
Propane
MASS SPECTRUM
+4₂
20
30
m/z
NIST Chemistry WebBook (https://webbook.nist.gov/chemistry)
40
50
40
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Login AMMcGraw Hill C IT Central Authe OWLv2 | Assig a. Which compound will have the higher boiling point? Br₂ OICI P8 https://east.cengagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator assignment-take b. Which compound will have the higher boiling point? OCH4 OCC14 Submit Answer c. Which compound will have the higher boiling point? OCH, OH OCH, F Try Another Version Content 2 item attempts remaining OWLv21 On X [References] Cenarrow_forward11. 12. H3C Н Br CH3 а SOH КОН EtOH (CH3)3CO Karrow_forwardNON app.101edu.co Maps YouTube 2 Q C|OL|GA|C G b HO S A 2 H Command ...I S x CTO F E AX Gd Question 26 of 31 The chemical structure of a biologically relevant molecule is shown below. Which functional group is NOT present? A) alkene B) alkyl chain C) cyclohexane ring D) aromatic ring E) alcohol DII FB I- D Aav A MacBook Air 80 ← % 1:11:L:L; 5 W E R F H B D, V B N F2QEG V VG 1 CSUON ΕΕ J 00 * 8 M 9 DD 0 O L H command P t Done option III ? 1 E Gr K F F12 } + + Update : Submit delete returDarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY