
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
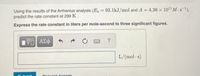
Transcribed Image Text:Using the results of the Arrhenius analysis (Ea = 93.1kJ/mol and A = 4.36 x 1011 M -s1),
predict the rate constant at 299 K.
Express the rate constant in liters per mole-second to three significant figures.
ΑΣφ
L/(mol s)
Reguest Answer
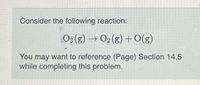
Transcribed Image Text:Consider the following reaction:
O3 (g) → O2 (g) + O(g)
You may want to reference (Page) Section 14.5
while completing this problem.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- The decomposition of a single compound at 330 K has a rate constant of 2.52 x 10-3 M-1 s-1. If the initial concentration of the reactant is 0.504 M, what is the concentration of the reactant after 26.30 seconds? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 12.3 and 120. and -123)arrow_forwardData in the table were collected at 540 K for the following reaction: CO(g) + NO₂ (g) → CO₂ (g) + NO(g) Initial concentration [CO] (mol/L) 5.1 x 10- 5.1 × 10-4 5.1 × 10-4 1.0 × 10-³ 1.5 × 10-³ [NO₂] 0.35 × 10-4 0.70 × 10-4 0.18 × 10-4 0.35 × 10-4 0.35 × 10-4 Initial rate (mol/L. h) 5.0 × 10-8 1.0 × 10-7 2.6 × 10-8 9.8 × 10-8 1.5 × 10-7arrow_forwardAt a certain temperature the rate of this reaction is second order in SO, with a rate constant of 4.02 M 's': 2S0, (g) → 2S0, (g) +0,(g) Suppose a vessel contains SO , at a concentration of 0.790 M. Calculate how long it takes for the concentration of SO, to decrease to 0.103 M. You may assume no other reaction is important. Round your answer to 2 significant digits.arrow_forward
- Consider this reaction: NH,OH (аq) — NH, (ад)+ н,о (ад) At a certain temperature it obeys this rate law. rate = (0.619 s)[NH,OH] S Suppose a vessel contains NH,OH at a concentration of 0.340M. Calculate the concentration of NH,OH in the vessel 2.60 seconds later. You may assume no 4 other reaction is important. Round your answer to 2 significant digits. ) M x10arrow_forwardThe rate of a reaction of Go is .457 mol/L•min. Calculate the rate for the appearance of Hu (mol/L•min)? 3Go + HuQw3 -> 3GoQw +Hu The rate of appearance of Ae is 0.777 mol/L•min. Determine the rate of disappearance of T (mol/L•min). 2 T + Ru2 + 2 Ae3Ge -> 2 TRuGe + 6 Aearrow_forwardA certain reaction is first order in N, and second order in H,. Use this information to complete the table below. Round each of your answers to 3 significant digits. N] [+.] H, initial rate of reaction x10 0.974 M 1.96 M 7.00 x 10 M/s 0.974 M 0.604 M | MIs 1.32 M 1.44 M Mls M/sarrow_forward
- 8 H chemPad H XX LE # Supporting Materials Periodic Table Additional Materials W 17 - Su2... → Help Greek Constants and W The reaction of fluorine and chlorine dioxide, shown below, is first order with respect to fluorine and first order with respect to chlorine dioxide. Write the rate law for this reaction. (Rate expressions take the general form: rate = k. [A]a. [B]b.) F₂(g) + 2 CIO₂ (g) → 2 FCIO₂(g) Factors G b * webassign.net 8 Supplemental Data L a ▸ Garrow_forwardConsider the initial-rate data at a certain temperature in the table for the reaction described by 2NO₂(g) +Q₂(g) →N₂0₂g+%₂8 Determine the value and units of the rate constant, k. k = [NO2] (M) 0.650 1.10 1.76 Units: [03] (M) 0.800 0.800 1.40 Initial rate (M/s) 3.25 x 104 5.50 X 104 15.40 X 10arrow_forwardThe synthesis of ammonia obeys the following reaction: N2(g) + 3H2(g) → 2 NH3(g) N2(g) concentration measurements were taken at different times and plotted. 1) What is the average reaction rate of NH3(g) between 0 and 3 hours? (Attention, the graph is in "hour" and we want a speed in "mole/L s") 2) What is the instantaneous rate of reaction of NH3(g) at the 1st hour? 3) What is the general speed between 0 and 3 hours?arrow_forward
- Consider this reaction: 2N₂O5 (g) → 2N₂O4 (g) + O₂(g) At a certain temperature it obeys this rate law. rate = (0.0317 M¹-s¯¹) [₂0₂] S Suppose a vessel contains N₂O5 at a concentration of 0.440 M. Calculate the concentration of N₂O5 in the vessel 480. seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M x10 ? X Sarrow_forward- 1 At a certain temperature the rate of this reaction is second order in H,CO, with a rate constant of 0.00683 M - 1 S. : Н,СО, (аq) — н,0 (ад)+со,(аq) Suppose a vessel contains H,CO, at a concentration of 1.01 M. Calculate the concentration of H,CO, in the vessel 580. seconds later. You may assume no 3. other reaction is important. Round your answer to 2 significant digits. | M x10 ?arrow_forwardWhat is the half life of the following reaction, given the following set of conditions (Hint: observe the units for the rate constant to determine the order of the reaction!): 2 HI (g) → H2 (g) + I2 (g) Initial concentration of HI = 1.00 M k = 1.2 x 10-3 M-1 s-1 Select one: a. 420 s b. 240 s c. 580 s d. 830 sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY