
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
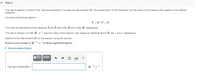
Transcribed Image Text:Part C
The rate of reaction in terms of the "rate law expression" includes the rate constant (k). the concentration of the reactants, and the orders of the reaction with respect to the different
reactants.
Consider the following reaction:
A +B»C+D
The initial concentrations of the reactants A and B are 0.380 M and 0.280 M., respectively.
The rate of reaction is 0.080 M -s1, and the orders of the reaction, with respect to reactants A and B, are 1 and 2. respectively.
Determine the rate constant (k) for the reaction using the rate law.
Express your answer in M 2.s 1 to three significant figures.
• View Available Hint(s)
The rate constant(k) =
M 2.s-1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The rate constant for a reaction is found to be 0.15 M-1s-1. If the initial concentration of the reactant is 0.20 M, how long (in seconds) does it take for the concentration to decrease to 0.15 M?arrow_forwardThe following initial rate data are for the reaction of ICI with hydrogen: 2 ICI + H2- →I2 + 2 HCI Experiment [ICI],, M [H2]o, M Initial Rate, M s-1 1 0.339 7.80x10-2 |3.63х10-3 0.678 7.80x10-2 7.26x10-3 3 0.339 0.156 1.45x10-2 14 0.678 0.156 2.90x10-2 Complete the rate law for this reaction in the box below. Use the form k[A]m[B]" , where '1' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n. Rate = From these data, the rate constant is M-2s-1.arrow_forwardA certain reaction is second order in N, and first order in H,. Use this information to complete the table below. Round each of your answers to 3 significant digits. [N] [H.] initial rate of reaction 1.89 M 2.21 M 6.00 x 10° M/s I M/s 1.89 M 0.910 M 2.37 M 1.76 Marrow_forward
- Units of M s-1 pleasearrow_forwardThe following data were obtained for the hypothetical reaction: The proper units of the rate constant should be: The data necesary is in the imagearrow_forwardPart C The rate of reaction in terms of the "rate law expression" includes the rate constant (k), the concentration of the reactants, and the orders of the reaction with respect to the different reactants. Consider the following reaction: The initial concentrations of the reactants A and B are 0.200 M and 0.350 M, respectively. The rate of reaction is 0.060 M.s -1, and the orders of the reaction, with respect to reactants A and B, are 1 and 2, respectively. Determine the rate constant (k) for the reaction using the rate law. -2 -1 Express your answer in M S to three significant figures. ► View Available Hint(s) The rate constant (k) = 1.78 Submit for Part C for Part Cdo for Part Provide Feedback Previous Answers X Incorrect; Try Again; 3 attempts remaining redo for part C reset for Part C keyboard shortcuts for Part C help for Part C M-2.s-1 A+B+C+Darrow_forward
- Determine order of reaction with aspect To A show reasoning determine order of reaction with respect to B showing reason write rate of reaction what are units of rate constant with reasoning Determine value of Karrow_forward23 II. An experiment followed the decomposition. of nitregen dioxide to nitregen monoxide & oxygen gas. Given the following data, what order is the reaction for nitrogen dioxide? Given an initial concentration of nitrogen dioxide of 0.0400 M, what's the concentration of this reactant after a half hour? show all grapers & calcs to determine answers. (sec) Time |[NO₂] (M) 0.0 50.0 100.0 200.0 300.0 0.01000 0.00787 0.00649 0.00481 0.00380 M G 2 XX 6.5 Hoa X019 201 2(x) = 2 (1.86) (3 (9)arrow_forwardA certain reaction is second order in N, and first order in H,. Use this information to complete the table below. Round each of your answers to 3 significant digits. [N] H,] initial rate of reaction x10 0.929 M 1.05 M 95.0 M/s olo 0.436 M 1.05 M |M/s Ar 0.165 M 5.93 М ||M/sarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY