
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
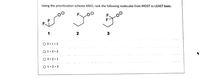
Transcribed Image Text:Using the prioritization scheme ARIO, rank the following molecules from MOST to LEAST basic.
F
F.
1
2
3
O 3 > 1 > 2
O 1> 3 > 2
O 3 > 2 > 1
O 1> 2 > 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the molecules shown below, draw the acid dissociation reaction. If a conjugate base has resonance structures, draw all valid resonance structures. A H* + H. - H* + CF3 H. C H* + Identify the stronger acid in each of the pairs below; provide an explana for each pair. A & B B &C A& Carrow_forwardRank and EXPLAIN WHY pleasearrow_forwardNitrogen atoms are known to have basic (proton acceptor) properties. Consider the following nitrogen heterocycle and the nitrogen atoms labelled 1- 3. N H₂N Which (a-f) ranks the nitrogen atoms in order of most basic to least. O a 1,2,3 O b. 1,3,2 O c. 2,1,3 O d. 2,3,1 O e. 3, 1, 2arrow_forward
- 6. Based on the following pKa values, which do you think is more important in determining inductive effects: electronegativity or distance from the reactive acidic hydrogen? Explain in 1-3 complete sentences. ОН pKa = 2.68 ОН pKa = 3.85 ОН CI pKa = 2.95 alon pKa = 3.97arrow_forwardCircle the most acidic proton out of the molecules below and explain your answer briefly. II II II H3C-S-OH H3C-S-OH F3C-S-OH II O=S=0arrow_forwardUse the information in the pK table to rank the molecules in order of increasing basicity. For example, select "1" in the second column for the weakest base a and "2" for the next weakest base and so on. CI CH,NH NH3 HSO4 Molecules (Choose one) (Choose one) (Choose one) (Choose one) X Ś CH₁ 48 NH3 H₂ CH3NH₂ 33 H₂O CH₂OH 38 36 15.7 15.5 pKa CH3NH 10.6 CH₂ SH HCN + NH table H₂S CH,CO,H 10.4 9.4 9.2 7.00 4.76 HF + H₂O* HC1 3.17 CH₂OH H₂SO4 -3.0 -7 -9 HBr -1.7 -2.2 ? 00. 18 Ararrow_forward
- Can someone help me find the most acidic hydrogen that is marked with an arrow out of these two compounds? Thank you!!arrow_forwardWhat is/are the most acidic proton(s) in the moluecle below? Select the answer where the most acid proton(s) is/are circled. H A B C H₂ A H₂ CH3 H₂ B H₂ CH3 H H₂ O=C H₂ H₂ с -CH3 CH3 H₂ D QH3arrow_forwardIdentify the most acidic hydrogen in the following compound. H3C- 04 02 0 1 O 3 O CH2-C-CH2-CH3 2 3 4arrow_forward
- 1. Rank the acidity of the hydrogen atoms shown on the following molecules (1= most acidic, 4=least acidic). Be sure to provide the reasoning for your choices. H H H. A Rank: 1. 2. 3. 4. Reasoning: o= 0=arrow_forwardRank the molecules in decreasing (strongest to weakest) acidity. Include ALL resonance structures with arrows and explain reasoning for rank.arrow_forwardc5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY