
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
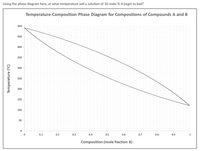
Transcribed Image Text:Using the phase diagram here, at what temperature will a solution of 36 mole % A begin to boil?
Temperature-Composition Phase Diagram for Compositions of Compounds A and B
500
450
400
350
300
250
200
150
100
50
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
Composition (mole fraction B)
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Molecular bromine is 24 percent dissociated at 1600 K and 1.00 bar in the equilibrium Br₂(g) ⇒ 2Br(g). Calculate (a) Kp, (b) A,Gº, (c) K₂ at 2000 K given that A₁HⓇ = +112 kJ mol-¹ over the temperature range.arrow_forward(c)Explain the meaning of Azeotrope and their effect in distillation process. Describe how an Azeotrope of ethanol and water can be separated.arrow_forward1. Use the Chapman-Enskog theory and Fuller correlation to estimate the diffusion of hydrogen in nitrogen at 21°C and 2 atmospheres.arrow_forward
- Please solve correctly, will provide helpful ratings. Thank youarrow_forwardI already have the answer and provide it. Show work pleasearrow_forward(a) In the recent years, the extractive heterogeneous-azeotropic distillation (EHAD) method gave great results in this field of separation (Toth et al., 2018). Acetone (1) and chloroform (2) mixture forms an azeotrope at temperature about 65 °C (338.15 K) and pressure of 101.325 kPa. The composition of azeotrope was found to be 0.335. i. Determine the value of GE/RT.arrow_forward
- EVALUATION OF MASS DIFFUSIVITY Find the diffusivity of CO, in N2 at 30°C and 740 mm Hg. Find the diffusivity of benzene vapor in air at 600C and 120 kPa Find the diffusivity of isopropyl alcohol in liquid water at 45°Carrow_forwardty, final question isarrow_forwardButane (CH) is burned with 25% exceed air during a combustion process. Assuming complete combustion, determine the following for this reaction: a) air-fuel ratio, fuel-air ratio. equivalence ratio and Can part of the fuel not be burned? Show the details of your solution, including the balances of the actual and ideal reactionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The