
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Help please :)
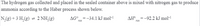
Transcribed Image Text:The hydrogen gas collected and placed in the sealed container above is mixed with nitrogen gas to produce
ammonia according to the Haber process shown below.
N,(g) + 3 H,(g) = 2 NH,(g)
AG° = -34.1 kJ mol-!
AH® = -92.2 kJ mol-1

Transcribed Image Text:Using the data in the table below and the enthalpy of reaction, AH°, calculate the approximate bond
energy of the nitrogen–hydrogen bond in ammonia.
Approximate
Bond Energy
(kJ mol')
Bonds
N-H
???
H -H
430
NEN
960
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- HO- 1) DMSO, (COCI)2 2) Et,N :? Editarrow_forward1 4 6 7. 8 9. 10 -10 An aqueous solution at 25 °C has a H,O' concentration of 6. x 10 "M. Calculate the OH concentration. Be sure your answer has 1 significant digits. | M Subm Continue © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centarrow_forward(4c-201) Copy the following equation, and label the Bronsted-Lowry acid, its conjugate base, the Bronsted-Lowry base, and its conjugate acid. HNO3 + H2O → H30* + NO3¬ For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph Arial 14px A v ... In X2 田由田図 EX: ABC Ť {;} > >arrow_forward
- Select one for each boxarrow_forwardCtrl Caps Shift M Inbox (1,543)-ftantill@udeledu x Mail-Francesca A Tantillo-Out x Homepage-CHM150-251 Chen X ← C - с app.101edu.co Question 19 of 20 Vitamin C (ascorbic acid, CeHsO6, 176.12 g/mol) can be measured by redox titration with iodine solution. lodine is reduced to iodide ion, while ascorbic acid is oxidized to dehydroascorbic acid (C6H6O6). The iodine solution is usually made in the presence of iodide ion, forming the more stable triiodide ion, Is, giving the following overall reaction CeHsOc(aq) + Is (aq) + H₂O(1)→ CHO(aq) + 31-(aq) + 2H+(aq) The titration is carried out in the presence of starch, which forms a dark blue complex with the excess iodine when the endpoint is reached. Ascorbic acid can be used to standardize the iodine solution. A 0.315 g sample of ascorbic acid was titrated with iodine solution, requiring 30.2 mL to reach the endpoint. Calculate [ls], the molar concentration of Is ion in the solution. O L Tab En Esc 79°F Sunny ! 1 Q A N FI @ 2 W S F2 X # 3 E D…arrow_forwardTrial 1 Trial 2 Trial 3 Initial burette reading (mL) 2.29 1.41 1.95 Molarity of NaOH (M) 0.100 0.100 0.100 Volume of vinegar sample (mL) 5.00 5.00 5.00 Final burette reading (mL) 50.37 49.39 49.84 Table 2. Titration data Trial 1 Trial 2 Trial 3 Initial burette reading (mL) 2.29 1.41 1.95 Molarity of NaOH (M) 0.100 0.100 0.100 Volume of vinegar sample (mL) 5.00 5.00 5.00 Final burette reading (mL) 50.37 49.39 49.84 Expected color at end point Volume of NaOH used (mL) 48.08 47.98 47.89 Compute for the ff: a. Average moles of acetic acid (mol)? b. Average molarity of acetic acid (M)? c. Average molarity of acetic acid (M)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY