
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
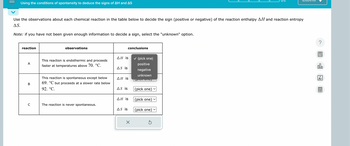
Transcribed Image Text:Using the conditions of spontaneity to deduce the signs of AH and AS
Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy
AS.
Note: if you have not been given enough information to decide a sign, select the "unknown" option.
reaction
observations
conclusions
ΔΗ is
A
This reaction is endothermic and proceeds
faster at temperatures above 70. °C.
AS is
✓ (pick one)
positive
negative
unknown
B
This reaction is spontaneous except below
69. °C but proceeds at a slower rate below
92. °C.
ΔΗ is
\PICK O
AS is
(pick one)
ΔΗ is
(pick one)
C
The reaction is never spontaneous.
AS is
(pick one)
☑
000
18
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider the reaction2CO2(g) + 5H2(g)C2H2(g) + 4H2O(g)Using the standard thermodynamic data in the tables linked above, calculate G for this reaction at 298.15K if the pressure of each gas is 19.41 mm Hg.ANSWER: kJ/molarrow_forwardhelp a girl outarrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy A.S. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A B C observations The reverse of this reaction is always spontaneous but proceeds slower at temperatures below -26. °C. This reaction is spontaneous except above -42. °℃. This reaction is endothermic. AH is AS is conclusions AH is AS is AH is AS is X (pick one) (pick one) (pick one) (pick one) (pick one) (pick one)arrow_forward
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions AH is (pick one) The reverse of this reaction is always spontaneous but proceeds faster at A temperatures above 25. °C. AS is (pick one) v This reaction is spontaneous except below AH is (pick one) 63. °C but proceeds at a faster rate above 117. °C. AS is (pick one) v AH iS This reaction is always spontaneous, but proceeds faster at temperatures above (pick one) v C 115. °C. AS is (pick one)arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy A.S. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A B с observations This reaction is spontaneous only above 30. °C but proceeds at a slower rate below 199. °C. This reaction is spontaneous only below 19. °C. This reaction is exothermic and proceeds slower at temperatures below 60. °C. conclusions AH is AS is AH is AS is AH is AS is X (pick one) (pick one) (pick one) ✓ (pick one) (pick one) (pick one) Śarrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A B C observations This reaction is slower below - 1. °C than above. This reaction is spontaneous only below -3. °C. The reaction is never spontaneous. AH is AS is conclusions AH is As is AH is AS is X ✓ (pick one) î positive negative unknown (pick one) (pick one) (pick one) (pick one) Ś ↑ îarrow_forward
- Calculate the reaction enthalpy for the decomposition of hydrogen peroxide, 2H2O20 H26) + O26) 2H2) + O216 2H2O0 + O26 from the following data: H2O20; AH°RXN = -187.8 kJ - 2H2O0; AH°Rxn = -571.6 kJarrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A The reverse of this reaction is always spontaneous. As is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only below 20. °C. As is (pick one) с This reaction is spontaneous except below 85. °C but proceeds at a slower rate below ΔΗ is (pick one) 129. °C. As is (pick one) ×arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy ▴ and reaction entropy as. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A B C observations The reverse of this reaction is always spontaneous. This reaction is spontaneous except above 64. °C. This reaction is always spontaneous, but proceeds faster at temperatures below 77. °C. AH is AS is conclusions AH is AS is AH is AS is x (pick one) (pick one) (pick one) (pick one) (pick one) (pick one)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY