
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
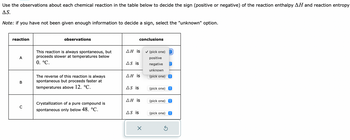
Transcribed Image Text:Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy
AS.
Note: if you have not been given enough information to decide a sign, select the "unknown" option.
reaction
A
B
C
observations
This reaction is always spontaneous, but
proceeds slower at temperatures below
0. °C.
The reverse of this reaction is always
spontaneous but proceeds faster at
temperatures above 12. °C.
Crystallization of a pure compound is
spontaneous only below 48. °C.
AH is
As is
ΔΗ is
AS is
conclusions
AH is
As is
X
✓ (pick one)
positive
negative
unknown
(pick one)
(pick one)
(pick one)
(pick one)
S
î
û
î
↑
↑
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Try Again Your answer is incorrect. Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A reaction B: Your answer is incorrect. B с observations This reaction is always spontaneous, but proceeds faster at temperatures above 9. °C. This reaction is spontaneous except below -20. °℃ but proceeds at a slower rate below 73. °C. Crystallization of a pure compound is spontaneous only below - 16. °C. AH is AS is conclusions AH is AS is AH is AS is X negative ↑ positive ↑ positive î negative ↑ negative ↑ negative ↑ 5arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy A.S. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction B C observations This reaction is always spontaneous, but proceeds faster at temperatures below 121. °C. Crystallization of a pure compound is spontaneous only below 109. °C. This reaction is exothermic and proceeds faster at temperatures above 34. °C. conclusions AH is AS is AH is AS is AH is AS is X (pick one) ✓ (pick one) (pick one) (pick one) (pick one) (pick one) Śarrow_forwardOne mole of an ideal gas undergoes the cycle, A⟶B⟶C⟶A, as shown in the graph. For this gas, CV = 1.5R. Step C⟶A is isothermal and reversible. 1) Calculate W for each step in the process.arrow_forward
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy A.S. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A B C observations The reverse of this reaction is always spontaneous but proceeds slower at temperatures below -26. °C. This reaction is spontaneous except above -42. °℃. This reaction is endothermic. AH is AS is conclusions AH is AS is AH is AS is X (pick one) (pick one) (pick one) (pick one) (pick one) (pick one)arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A B C observations This reaction is always spontaneous, but proceeds faster at temperatures below 140. °C. The reaction is never spontaneous. This reaction is spontaneous only above 129. °C but proceeds at a slower rate below 192. °C. AH is AS is conclusions AH is AS is AH is AS is X ✓ (pick one) positive negative unknown (pick one) î (pick one) (pick one) C Ś ↑ (pick one) îarrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy A.S. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A B с observations This reaction is spontaneous only above 30. °C but proceeds at a slower rate below 199. °C. This reaction is spontaneous only below 19. °C. This reaction is exothermic and proceeds slower at temperatures below 60. °C. conclusions AH is AS is AH is AS is AH is AS is X (pick one) (pick one) (pick one) ✓ (pick one) (pick one) (pick one) Śarrow_forward
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A B C observations This reaction is slower below - 1. °C than above. This reaction is spontaneous only below -3. °C. The reaction is never spontaneous. AH is AS is conclusions AH is As is AH is AS is X ✓ (pick one) î positive negative unknown (pick one) (pick one) (pick one) (pick one) Ś ↑ îarrow_forwardD Try Again Your answer is incorrect. Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction A • reaction A: Your answer is incorrect. • reaction C: Your answer is incorrect. B с observations The reverse of this reaction is always spontaneous. This reaction is exothermic and proceeds slower at temperatures below -26. °C. This reaction is always spontaneous, but proceeds faster at temperatures above 139. °C. AH is AS is conclusions AH is As is AH is AS is X unknown negative ŷ negative ↑ unknown î unknown ↑ positive î Sarrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A The reverse of this reaction is always spontaneous. As is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only below 20. °C. As is (pick one) с This reaction is spontaneous except below 85. °C but proceeds at a slower rate below ΔΗ is (pick one) 129. °C. As is (pick one) ×arrow_forward
- A cvg.cengagenow.com Flipgrid | 50ffc. GRLContent Netflix Favorites My Home 3-Day General.. Netflix Content [Review Topics] [References] 2req Use the References to access important values if needed for this question. 2req The free energy change for the following reaction at 25 °C, when [H*] = 7.21×10³ M and [Hg²*]=1.17 M, is 178 kJ: 2req 2H*(7.21×10-3 M) + Hg(1)→ H,(g) + Hg²*(1.17 M) AG= 178 kJ What is the cell potential for the reaction as written under these conditions? s 2req Answer: V ts 2req Would this reaction be spontaneous in the forward or the reverse direction? ots OM) pts 2reg Submit Answer Retry Entire Group 9 more group attempts remaining pts M) ptsarrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions AH is A This reaction is always spontaneous, but proceeds slower at temperatures below 45. °C. AS is ΔΗ is This reaction is spontaneous only above 150. °C but proceeds at a faster rate above 180. °C. AS is ΔΗ is This reaction is exothermic and proceeds slower at temperatures below 67. °C. AS is X C (pick one) (pick one) (pick one) (pick one) ✓ (pick one) (pick one) Ś ?arrow_forwardGive answer second question with explanationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY