
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
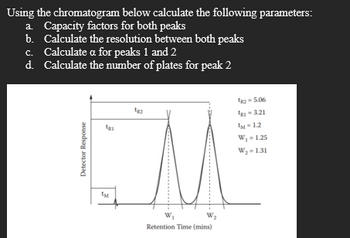
Transcribed Image Text:Using the chromatogram below calculate the following parameters:
a. Capacity factors for both peaks
b. Calculate the resolution between both peaks
c. Calculate a for peaks 1 and 2
d. Calculate the number of plates for peak 2
Detector Response
W₂
Retention Time (mins)
1-5.06
02-321
M-12
W₁ = 1.25
W2-1.31
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- The same polar compound is gas chromatographed on an SE-30 (very nonpolar) column and then on a Carbowax 20M (very polar column). I low will K=cS/cM vary between the two columns?arrow_forwardFrom the data in Problem 26-14, calculate for species C and D) (a) the resolution. (b) the length of column necessary to separate the two species with a resolution of 2.5.arrow_forwardMass spectrometry is an extremely versatile detection system for GC. However, interfacing an HPLC system to a mass spectrometer is a much more difficult task. Describe the major reasons why it is more difficult to combine HPLC with mass spectrometry than it is to combine GC with mass spectrometry.arrow_forward
- 1) Study the chromatograph (below) of a mixture of Compounds A and B, run on the GCs in the teaching labs at CU Boulder. Compound A has the shorter retention time. STAAT 61 1.11 227 RT TYPE AREA XXXX XXXX XXXX AREAS 0.009 55874 44.117 ARIHT 0.61 XX XX XX 1.11 2.27 XX XX XX TOTAL AREA=XX MUL FACTOR=XX 1. What is the retention time of compound A? Compound B? 2. Which compound is present in a larger amount? 3. Which compound has the lower boiling point? 4. What would happen to the retention times of compounds A and B if the column temperature were raised? 5. You suspect that compound B is octane. What can you do to provide supporting evidence for this hypothesis?arrow_forward13. Electrospray time-of-flight mass spectrometry was used to analyze the eluate from a high-performance liquid chromatography separation. The mass spectrum of one chromatographic peak, containing a protein of unknown molecular mass, displays MH peaks at m/z = 5 654.208, 5 277.326, 4 947.590, 4 656.613, 4 397.990, and 4 166.576. Find the average molecular mass (M) of the neutral protein and its standard deviation.arrow_forwardInternal standard. A mixture containing 12.8 mM analyte (X) and 44.4 mM standard (S) gave chromatographic peak areas of 306 for X and 511 for S. A second solution containing an unknown quantity of X plus 55.5 mM S had peak areas of 251 for X and 563 for S. Find [X] in the second solution.arrow_forward
- The following chromatogram is obtained for a mixture of substances X and Y. Calculate the resolution of the two peaks. 10 11 12 13 14 Tim e (min) O A. 0.8 О В. 1.7 O C. 1.2 O D. 3.4 O E. 0.4 Instrum ent response in arbitrary unitsarrow_forwardThe following chromatogram is obtained for a mixture of substances X and Y. Calculate the separation factor. Y 1 10 11 12 13 14 Tim e (min) O A. 1.4 O B. 0.4 O C. 0.8 O D. 1.2 O E. 2.1 Instrument response in arbitrary unitsarrow_forwardFor quantitative analysis with gas chromatography: O the area of a peak reported by the instrument for a compound is proportional to the quantity of that compound. O the calculated response factor value for a compound is proportional to the quantity of that compound. O the retention time reported by the instrument for a compound is proportional to the quantity of that compound. O the peak height reported by the instrument for a compound is inversely proportional to the quantity of that compound. O the peak shape reported by the instrument for a compound is proportional to the quantity of that compound.arrow_forward
- All types of chromatography depend on partitioning a sample between a stationary phase and a mobile phase. Identify the stationary phase and mobile phase for each of the following types of chromatography. -TLC -GCarrow_forward1 24) The method of internal standard was used to analyze and measure the concentration of chemical X by GC A known mixture of compounds X and Y (internal standard) gave the following chromatographic results: Compound X Y Concentration (M) 6.3 x 10-8 2.0 x 10-7 a) 2.0 x 10-6 M b) 8.4 x 10-7 M c) 6.6 x 10-7 M d) 4.1 x 10-7 M e) 2.9 x 10-7 M Peak Area 395 787 Then, an unknown was analyzed as follows. 3.0 mL of unknown X was treated with 0.100 mL of 1.6 x 10-5 M Y and the mixture diluted to 10.0 mL final volume. Peak areas were 633 (for X) and 520 (for Y). What is the concentration of X in the unknown. (The original 3.00 sample?)arrow_forwardThe figure below shows a portion of a GC chromatogram for a mixture of two aromatic compounds labeled A and B. The separation employed a 2.50 meter packed column under isothermal conditions (90 °C) and a flow rate of 10 mL/min. Detector Signal 300.0 250.0 200.0 150.0 100.0 50.0 0.0 1.0 1.1 Unretained 1.5 time (min) Find the times that components A and B spend in the stationary phase. 2.0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole