
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
1
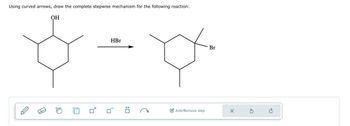
Transcribed Image Text:Using curved arrows, draw the complete stepwise mechanism for the following reaction:
OH
HBr
:0
Add/Remove step
Br
X
Ś
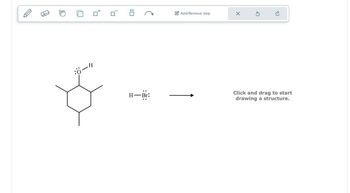
Transcribed Image Text:Η
:Ö-H
e
H-Br:
Add/Remove step
X
S
Ć
Click and drag to start
drawing a structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- B E *** B B ܘ C NaOH ܚ ܐ Earrow_forward5. Identify the following as a physical or chemical change: (a) water boils at 212 °F (b) applying an electric current to water causing the evolution of a gas (c) some metal statues obtain a green color after years of exposure to the atmosphere (d) evaporation of water from a saline solution leaves a solid salt residuearrow_forward100% nus (Alt+/) I 21 1 1 DATA AND CALCULATIONS Pressure Volume (kPa) (mL) 5.8 mL 191.30 10.8 103.47 13.3 84.13 15.8 71.52 18.3 62.57 20.8 55.66 PROCESSING THE DATA/CONCLUSION 1. If the volume is doulbled from 5.0 mL to 10.0 mL, w pressure? Slhow the pressure values in your answer.arrow_forward
- a) An acetaminophen suspension for infants contains 80 mg/ 0.80 mL suspension. The recommended dosage is 15 mg/kg body weight. How many mL of this suspension should be given to an infant weighing 6.5 kg? b) A runner wants to run 10.0 km. She knows that her running pace is 3.5 m s- 1. How many minutes must she run?arrow_forward(7.211*10^5)*(5.3413*10^4)/4.09*10^-7arrow_forward% error = (experimental value - accepted value) x 100 Accepted value Siver 10.06cm^3 -10.49cm^3 *100 / 10.49 Is this correct ?arrow_forward
- 2. Lead metal can be extracted from a mineral called galena, which contains 86.6% lead by mass. A particular ore contains 68.5% galena by mass. A particular ore contains 68.5% galena by mass. If the lead can be extracted with 92.5% efficiency, what mass of ore is required to make a lead sphere with a 5.00 cm radius?arrow_forwardEvery year Every second (1 year 365 days).arrow_forwardhboard My Home C OWLV2 | Online teaching and learning resource from Cengage Lear. [References] INTERACTIVE EXAMPLE Converting Units: Any Unit to Another Unit Gasoline at a gas station in Canada costs 1.13 Canadian dollars (CAD) per liter. If the exchange rate between US and Canadian dollars is 1 US dollar (USD) = 1.15 CAD, what is the cost of the gasoline in US dollars per gallon? 1 gallon = 3.78 L. USD/gal Show Tutor Stenearrow_forward
- Not sure what I am doing wrong here...arrow_forwardQ: (4.80 x 10^{-107}) X (5.00 x 10^{-11})= a 2.40 x 10^{-96} b 24.0 x 10^{-96} c 2.40 x 10^{-118} d 2.40 x 10^{-117}arrow_forwardColonel James Rhodes is prescribed to take 3 pills per day (pill/day) of a specific medication. If each pill contains 34 mg of the active dose, how many grams of the drug will he receive each week (g/week)? Show all steps using dimensional analysisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY