
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Which of the statements below are true of the substance represented in the diagram?
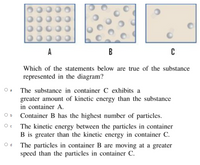
Transcribed Image Text:A
B
Which of the statements below are true of the substance
represented in the diagram?
O a
The substance in container C exhibits a
greater amount of kinetic energy than the substance
in container A.
O b
Container B has the highest number of particles.
The kinetic energy between the particles in container
B is greater than the kinetic energy in container C.
O d
The particles in container B are moving at a greater
speed than the particles in container C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student add 15g of baking soda to 10g of acetic acid in a beaker. A chemical reaction occurs and a gas is given off. After the reaction, the mass of the products remaining in the beaker is 23g. Has mass been conserved in this reaction? Explainarrow_forwardPart A and Barrow_forwarda chemist has 19.50g alcohol. what is the volume of the sample, what additional information is needed in order to calculate the volume?arrow_forward
- Which of the following best describes the system depicted in the diagram? Mixture of elements Element Compound Mixture of compoundsarrow_forwardFor each box, examine the blocks attached to the balances. Based on their positions and sizes, determine which block is more dense (the dark block or the lighter-colored block), or if the relative densities cannot be determined. (Think carefully about the information being shown.)arrow_forwardGaseous butane (CH;(CH,),CH;) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,O). If 27.7 g of water is produced from the reaction of 56.95 g of butane and 114.1 g of oxygen gas, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it.arrow_forward
- Which of the following is best characterized as a heterogeneous mixture? Group of answer choices (a) Air (b) Sandy Dirt (c) An aqueous solution of NaCl (d) Electrum (an alloy of gold and silver) (e) Clear Diamondarrow_forwardA student adds 15g of baking soda to 10 g of acetic acid in a beaker. A chemical reaction occurs and a gas is given off. After the reaction, the mass of the products remaining in the beaker is 23g. Has mass been conserved in this reaction? Explainarrow_forwardA chemist has a 19.50 g sample of drinking alcohol (ethyl alcohol). What is the volume of the sample?What additional information does the chemist need in order to calculate the volume?arrow_forward
- Copper wire has a diameter of 2.00mm. What length of this wire containes 1.00 mol copper?arrow_forwardgive a particulate-level explanation of why ice (solid water) is less dense than liquid water?arrow_forwardA standard aspirin tablet contains 325 mg of aspirin, which has the molecular formula C9H8O4. How many miles and molecules of aspirin are in 1 tablet?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY