
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
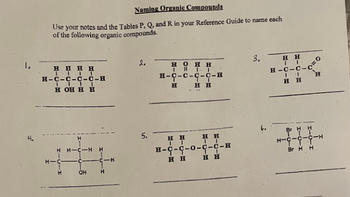
Transcribed Image Text:**Naming Organic Compounds**
Use your notes and the Tables P, Q, and R in your Reference Guide to name each of the following organic compounds.
1.
```
H H H
| | |
H—C—C—C—C—H
| | |
H OH H
```
2.
```
H O H
| || |
H—C—C—C—C—H
| | |
H H H
```
3.
```
H H
| |
H—C—C—C—C—O
|| |
H H
```
4.
```
H
|
H—C—C—C—H
| |
Br Br
```
5.
```
H H H
| | |
H—C—C—C—C—H
| | |
H OH H
|
H OH
```
---
**Interpretation of Structures:**
1. **Compound 1:**
- The structure shows a chain of four carbon atoms (butane) with an —OH group attached to the third carbon atom.
- This compound is 3-butanol.
2. **Compound 2:**
- The structure shows a chain of four carbon atoms (butane) with a double bond to oxygen (=O) at the second carbon, and an —OH group at the third carbon causing a presence of a hydroxyl group and a carbonyl group.
- This compound is 3-hydroxybutanal.
3. **Compound 3:**
- The structure shows a chain of four carbon atoms (butane) with a C=O (carbonyl) group at the end carbon atom forming an aldehyde.
- This compound is butanal.
4. **Compound 4:**
- The structure shows a chain of three carbon atoms (propane) with two bromine atoms attached to the second carbon atom.
- This compound is 2,2-dibromopropane.
5. **Compound 5:**
- The structure shows a chain of four carbon atoms (butane) with two —OH groups
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- HN-CH- H H. CH,CHCH, -C-N-CH–C–N–CH-C-N-CH-C–N–CHËO H H ČH-COO H. CH,OH CH3arrow_forwardOrganic chem questions I need helparrow_forwardWhich molecule is a complete organic molecule (i.e. all atoms are shown correctly)? A H. c=C–C= C–H H C H. C=C-C-C-H | H. H. C H. C- D H. нн . C С —С—Н C=C–C- C-H H H. ОН Н А I-0-I エーO I-Ú-I I-U-I I-U-I エー○arrow_forward
- 7. Name the following two acids in both delta as well as omega nomenclature: .COOH А. 1. A- 2. w- CH3-(CH2)5-CH=CH-(CH2)7-COOH В. 1. Д- 2. o–arrow_forwardOf the compounds below, HO CI | CI HO CI IV HO is the most acidic and || НО. CI НО. V CI CI ||| is the least acidic.arrow_forwardpredicting products and reactants organic chemistryarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY