
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Synthesis: in water react (see image), also label major/minor/trace products
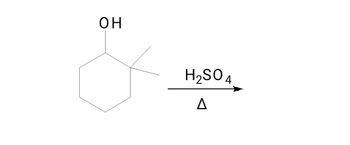
Transcribed Image Text:ОН
H₂SO4
Д
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student suggests that the molecule on the right can be made from a single starting molecule 1, under the conditions written above and below the arrow. Is the student correct? If so, draw 1 in the area below. If the student is not correct, just check the box under the drawing area. 1. NaOMe N म 1 2. H3O+ CH₂O Click and drag to start drawing a structure. : Garrow_forwardA student suggests that the molecule on the right can be made from a single starting molecule 1, under the conditions written above and below the arrow. Is the student correct? If so, draw 1 in the area below. If the student is not correct, just check the box under the drawing area. 1. NaOMe 1 CH3O 2. H₂O* N : ☐ A G Parrow_forwardte Bb Welcome, Kawtha... O Maps GE News Home [Review Topics)] [References] Draw structural formulas for the major organic product(s) of the reaction shown below. CH2CH3 stered FeBr3 Br2 You do not have to consider stereochemistry. If no reaction occurs, draw the organic starting material. • Remember to include all of the formal charges on the atoms of any nitro groups. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. opy aste Previous Next Email Instructor Save and Cengage Learning | Cengage Technical Supportarrow_forward
- Which molecule is a complete organic molecule (i.e. all atoms are shown correctly)? A H. c=C–C= C–H H C H. C=C-C-C-H | H. H. C H. C- D H. нн . C С —С—Н C=C–C- C-H H H. ОН Н А I-0-I エーO I-Ú-I I-U-I I-U-I エー○arrow_forwardI'm having trouble with my chemistry homework. For this question I chose "Pepto-Bismol – C7H5BiO4" The question is: Write out the chemical name of the compound you selected. For example, NaOH = sodium hydroxide. (Note: the example is not an antacid...it's a very strong base.) Please help mearrow_forwardPlease answer the following picture, thanks in advancearrow_forward
- Consider three molecules in the image below. Select two statements that are correct. H. H CH3 н :0: H H. H. H. H3C H3C-C- H3C H H CH3 H H H H. Ethyl acetate, CH;COOC,H5 5-tert-Butyl-m-xylene, C12H18 Aniline, CH,N If you can't see this image, please click here C12H18 has the weakest intermolecular forces. A pure sample of aniline has weaker intermolecular forces than a pure sample of 5-tert-Butyl-m-xylene. The dominant intermolecular forces in a pure sample of C6H,N is dipole-dipole forces The dominant intermolecular forces in a pure sample of Ethyl acetate, CH3COOC2H5 is dipole-dipole forces.arrow_forwardDraw a Lewis structure for the molecule below, showing all lone pairs. You may abbreviate any methyl groups as CH,. CH,CH(OH)CH,CCH Click and drag to start drawing a structure.arrow_forwardCondensed Structural Formula (Circle Functional Group) CH3-CH2-NH2 H3C-CC-CH3 CH3-SH H₂C-i-₁ HỌC-C-NH-CH3 2 Functional Group Amine 3 #val. e's 20 4 Lewis Structure H-C-C-N-H Molecular drawing HINNIC www 5 CHEM 10 2021-2022 мишт 4 6 Skeletal formula NH₂ Instructor Signature 7 Draw Vectors for Polar Bonds M Page 250 В Molecular Polarity? Yesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY