
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please don't provide handwritten solution .....
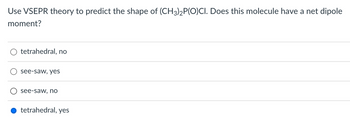
Transcribed Image Text:**Question:**
Use VSEPR theory to predict the shape of (CH₃)₂P(O)Cl. Does this molecule have a net dipole moment?
**Options:**
- ○ tetrahedral, no
- ○ see-saw, yes
- ○ see-saw, no
- ● tetrahedral, yes
**Explanation:**
The selected answer is "tetrahedral, yes."
The Valence Shell Electron Pair Repulsion (VSEPR) theory helps predict the 3D shape of molecules by considering electron pair repulsions. Here, dimethylphosphoryl chloride ((CH₃)₂P(O)Cl) is analyzed for its shape and dipole moment. The molecular shape is predicted to be tetrahedral due to the arrangement of substituents around the phosphorus atom. The presence of polar bonds and an asymmetric shape leads to a net dipole moment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How would someone be confident in an experimentally determined concentration? Explain the reasoning using technical terms.arrow_forwardWhat experimental technique should you use to determine the concentration of a colored solution? O measurements of volume & mass calorimetry O spectrometry O titration aarrow_forward12.1 g of goztic acid was dissolved in water weighing 87.9 G. The mass fraction (%) of acid in the solution was calculated.arrow_forward
- Can the solution be written out and solved step by step?arrow_forwardPlease don't provide handwrritten solution .....arrow_forwardMatch the following terms to their definitions: Aqueous solution, cation, centrifuge, decant, flame test, precipitate, qualitative analysis, supernate An insoluble solid formed when two solutions are mixed is called a ..... ? Group of answer choices Decant Precipitate Aqueous Solution Cation Centrifuge Flame Test Supernate Qualitative Analysisarrow_forward
- [References] Use the References to access important values if needed for this question. In the laboratory you dissolve 15.7 g of cobalt(II) fluoride in a volumetric flask and add water to a total volume of 125. mL. What is the molarity of the solution? Marrow_forward2) You are tasked with determining the barium content of a sample containing barium nitrate mixed with rubidium nitrate. The sample is weighed, dissolved into 50.0mg water, and treated with xs 0.500M sodium sulfate. The acid fully ionizes in this experiment. A white precipitate forms which is washed, filtered, and weighed multiple times, according to the data given below: Mass of sample 0.425g Mass of thoroughly dried filter paper 1.462g Mass of precipitate+filter after 1st drying 1.755g Mass of precipitate+filter after 2nd drying 1.699g Mass of precipitate+filter after 3rd drying 1.698garrow_forwardMasses : Ca = 40.08 g S= 32.07 g O= 16.00 g A 35.0 mL sample of CaSO4 was evaporated to dryness, leaving 0.967 g of residue. What was the molarity of the original solution? step by step pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY