
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Chemical Bonding -..
V Pennsylvania Acces...
Department of Hu..
Bvlgari Man In Blac..
O CHEMICAL REACTIONS
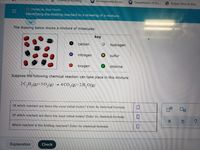
Identifying the limiting reactant in a drawing of a mixture
The drawing below shows a mixture of molecules:
key
carbon
hydrogen
nitrogen
sulfur
oxygen
chlorine
Suppose the following chemical reaction can take place in this mixture:
2C,H,(g)+50,(g) → 4 CO,(g)+2H,O(g)
Of which reactant are there the most initial moles? Enter its chemical formula:
Of which reactant are there the least initial moles? Enter its chemical formula:
Which reactant is the limiting reactant? Enter its chemical formula:
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Give detailed Solution with explanation needed...don't give Handwritten answerarrow_forwardThe drawing below shows a mixture of molecules: key carbon hydrogen nitrogen sulfur охудеn chlorine Suppose the following chemical reaction can take place in this mixture: CS,(9)+3 0,(9) → CO,(9)+2 SO,(g) Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula:arrow_forwardIdentifying the limiting reactant in a drawing of a mixturearrow_forward
- Consider the combustion reaction for octane (C,H2), which is a primary component of gasoline. 18 2 C3H13 + 25 0, → 16 CO, + 18 H,O How many moles of CO, are emitted into the atmosphere when 20.1 g C,H13 is burned? CO2 emitted: mol CO2arrow_forwardThe complete combustion of gasoline (C8H18) is best represented by which of the following equations Options: 2 C8H18 (l) + 25 O2 (g) → 16 CO2 (g) + 18 H2O(l) 2 C8H18 (l) + 17 O2 (g) → 16 CO (g) + 9 H2O (l) C8H18 (l) + O(g) → 8 CO2 (g) + 9 H2O (l) C8H18 (l) → 8 C (s) + 9 H2 (g)arrow_forwardProduct Composition and Reaction Stoichiometry Suppose SH2 and O2 react to produce H20, and a sulfur oxide which contains 40.05% sulfur by mass. Complete the reaction equation by entering the coefficients of the reactants and products and also the empirical formula of the sulfur oxide product. SH2 + H20 + All coefficients must be reduced to the smallest possible integers. Note that, while it is not usual to write coefficients of exactly 1, you must enter "1" in the response box, if that is the correct value. The formula for the sulfur oxide must be written with the S symbol first and O second. The usual subscription of numbers in the formula is to be inferred (e.g. the formula for water would be entered simply as "H2O")arrow_forward
- Hydrogen is manufactured on an industrial scale by this sequence of reactions: CH, (g) + H,0 (g) CO (g)+3 H, (g) K1 CO (g) + H,0 (g)= CO, (g)+H, (g) K2 The net reaction is: CH4 (g) +2 H,0 (g) CO, (g)+4H, (g) K Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K, and K,. If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator. Karrow_forward2NO(g) + O2(g) → 2NO2(g) You are provided with 4.12 molmol of nitrogen monoxide gas. Using the balanced chemical equation completed in Part A, determine how many moles of oxygen gas are needed to completely react with the nitrogen monoxide gas and how many moles of nitrogen dioxide are formed as a result? Ammonia and oxygen react to form nitrogen monoxide and water. Construct your own balanced equation to determine the amount of NO and H2O that would form when 2.80 molmol NH3 and 4.64 mol O2 react. Express the amounts in moles to two decimal places separated by a comma.arrow_forward2NaClO, --> 2NaCI + 30, Reactants combine into a large one Reactant breaks apart into smaller molecules A pure element swaps places with an element in a compound elements from two different compounds switch place a hydrocarbon reacts with oxygen to form carbon dioxide and waterarrow_forward
- Dilution Problems, Chemistry, Molarity & Concentration Examples,... M O O CHEMICAL REACTIONS Reaction sequence stoichiometry In the second step, iron(III) oxide and carbon monoxide react to form iron and carbon dioxide: Fe₂O3(s) + 3CO(g) → 2Fe(s) + 3 CO₂ (g) E crosoft O V Blast furnaces extra pure iron from the iron (III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide: 2C(s) + O₂(g) 2 CO (g) W Suppose the yield of the first step is 84.% and the yield of the second step is 63.%. Calculate the mass of oxygen required to make 3.0 kg of iron. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits. G Microsoft Microsoft 6.52.210... Explanation BO C Check 9,269 x10 믐 103 X OCT 24 ロ・ロ átv 2 3/5 DOZA Simrat 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility W E ol Ararrow_forwardWhat's #2?arrow_forwardDo not give handwriting solution.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY