
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
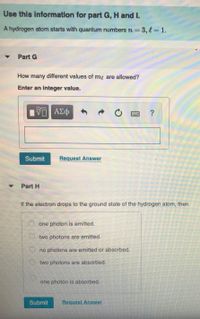
Transcribed Image Text:Use this information for part G, H and I.
A hydrogen atom starts with quantum numbers rn=3,13D1.
Part G
How many different values of my are allowed?
Enter an integer value.
ΑΣφ
Submit
Request Answer
Part H
If the electron drops to the ground state of the hydrogen atom, then
O one photon is emitted.
O two photons are emitted.
O no photons are emitted or absorbed.
O two photons are absorbed.
one photon is absorbed.
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the allowed quantum states(n,l, ml , ms )for a Hydrogen atom where the electron is in the n = 2 state? (p-orbital)arrow_forwardTwo different emission processes are shown in the energy level diagram. Which transition results in the emission of a photon with higher energy? n= 6 n= 4 n= 3 Energy O n=6 to n=2 n= 2 O n=4 to n=1 n= 1 Explain how you decided which transition emits a photon with a higher energy. Explain here..arrow_forwardNonearrow_forward
- Please help me with the following question, explain check that is correct, thank you sm!arrow_forwardit say's my answer is wrongarrow_forward(From experiment Atom.3.1 & Atom.3 SIR) Which of the electron transitions shown below is most likely to produce a red colored light? A n=1 D n=2 n=3 n=4 n=5 n=6 O A O B ODarrow_forward
- References Use the References to access important values if needed for this question. What would be the wavelength of radiation emitted from a hydrogen atom when an electron moves from the n= 3 to n = 2 energy level? In what region of the spectrum does this radiation lie? Wavelength nm %3D Region Submit Answerarrow_forwardThe energy level diagram below shows the allowed energy levels of an electron in an atom of Element X. Energy (J) | |- C B A -1.1x10-20 -2.3x10-20 -4.9x10-20 Calculate the wavelength of a photon emitted by the transition from C to A. marrow_forwardChoose one wavelength from the atomic emission spectrum of hydrogen in FIGURE 1. Knowing that all visible lines in the hydrogen emission spectrum have n(1) = 2, use the Rydberg/Bohr equations to determine E for the emission and n(2) for this electron transition.arrow_forward
- Q1: The results of a photoelectric experiment comparing two different metals (A and B) are shown below. A B Frequency of light In the experiment, the KE of the photoelectrons is plotted vs. the frequency of the light used for each of the metals. Which metal has the greater p? Group of answer choices: B/cannot be determined/A Photoelectron KEarrow_forwardHow do we solve this? What steps are being used and which equations/methods?arrow_forward● Calculate the frequency of the red light emitted by a neon sign with a wavelength of 710 nm. 4.10 × 1014 S-1 2.30 × 1014 S-1 5.05 × 1014 S-1 3.40 × 1014 S-1 4.26 × 1014 S-1 yellow green QUESTION 8 ► 2 QUESTION 7 8 285 LONGE Mannh Eugen BURE SHE THEREU W BUS 3 japanese and How many different values of my are possible in the 6d sublevel? 3 Insomni News Johann PANCAKE pag SLU 7arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY