
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
 for the necessary constants.
- Input the calculated pressure in the box provided.
\[
P = \hspace{5cm} \text{atm}
\]
2. **Ideal Gas Equation:**
- Use the ideal gas equation to calculate the pressure \( P \) under the same conditions.
- Input the calculated pressure in the box provided.
\[
P = \hspace{5cm} \text{atm}
\]](https://content.bartleby.com/qna-images/question/4cbada35-3122-4a6c-a25c-246bca0ae236/c3712587-b5d8-4409-a8db-f5b623655b75/dx4eknf_thumbnail.png)
Transcribed Image Text:**Van der Waals and Ideal Gas Calculations**
**Objective:**
Calculate the pressure \( P \) of 2.70 mol of \( \text{NH}_3 \) at 481 K in a 4.30 L vessel using two different methods: the van der Waals equation of state and the ideal gas equation.
**Instructions:**
1. **Van der Waals Equation:**
- Use the van der Waals equation to calculate the pressure \( P \).
- Refer to this [list of van der Waals constants](#) for the necessary constants.
- Input the calculated pressure in the box provided.
\[
P = \hspace{5cm} \text{atm}
\]
2. **Ideal Gas Equation:**
- Use the ideal gas equation to calculate the pressure \( P \) under the same conditions.
- Input the calculated pressure in the box provided.
\[
P = \hspace{5cm} \text{atm}
\]
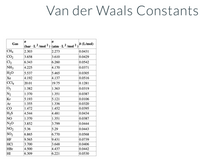
Transcribed Image Text:# Van der Waals Constants
The table below provides the Van der Waals constants for various gases. These constants are used in the Van der Waals equation for real gases which accounts for the volume occupied by gas molecules and the attractive forces between them.
| Gas | \( a \) (bar · L²/mol²) | \( a \) (atm · L²/mol²) | \( b \) (L/mol) |
|------|--------------------------|--------------------------|----------------|
| CH₄ | 2.303 | 2.273 | 0.0431 |
| CO₂ | 3.658 | 3.610 | 0.0429 |
| Cl₂ | 6.343 | 6.260 | 0.0542 |
| NH₃ | 4.225 | 4.170 | 0.0371 |
| H₂O | 5.537 | 5.465 | 0.0305 |
| Xe | 4.192 | 4.137 | 0.0516 |
| CCl₄ | 20.01 | 19.75 | 0.1281 |
| O₂ | 1.382 | 1.363 | 0.0319 |
| N₂ | 1.370 | 1.351 | 0.0387 |
| Kr | 5.193 | 5.121 | 0.0106 |
| Ar | 1.355 | 1.336 | 0.0320 |
| CO | 1.472 | 1.452 | 0.0395 |
| H₂S | 4.544 | 4.481 | 0.0434 |
| NO | 1.370 | 1.351 | 0.0387 |
| N₂O | 3.852 | 3.799 | 0.0444 |
| NO₂ | 5.36 | 5.29 | 0.0443 |
| SO₂ | 6.865 | 6.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- A bottle of N2O3(G) has a pressure of 0.475 atm. When the absolute temperature of the N2O3 (G) is tripled, the gas completly decomposes, producing NO2 (g) and NO(G). Calculate the final pressure of the gas mixture, assuming that the bottle volume does not change. Answer should be in atm.arrow_forwardexpain step by steparrow_forwardCalculate the pressure (in atm) of a 5.30 mol sample of nitrogen gas at 200.0 K in a 2.00 L container using the van der Waals equation. For nitrogen, a = 1.39 L²・atm/mol² and b = 0.0391 L/mol.arrow_forward
- Calculate the pressure (in atm) of a 4.50 mol sample of ammonia gas at 350.0 K in a 3.00 L container using the van der Waals equation. For ammonia, a = 4.17 L²・atm/mol² and b = 0.03712 L/mol.arrow_forwardReal gases can exist under conditions other than STP. True O Falsearrow_forwardCalculate the pressure of a 14.6 L container with 60.6 moles of hydrogen, at -14.5 degree C, assuming ideal conditions, and then calculate it assuming the gas is not behaving ideally. Compare the two values, and determine explain if the ideal gas law is appropriate to determine the pressure under these conditions. The van der waal constants for hydrogen are a=0.244(atm*L2 )/mol2 and b=0.0266 L/mol.arrow_forward
- Calculate the pressure (in atm) of a 2.10 mol sample of ammonia gas at 350.0 K in a 3.00 L container using the van der Waals equation. For ammonia, a = 4.17 L².atm/mol² and b = 0.03712 L/mol.arrow_forwardCalculate the pressure (in atm) of a 2.70 mol sample of ammonia gas at 350.0 K in a 3.00 L container using the van der Waals equation. For ammonia, a = 4.17 L²・atm/mol² and b = 0.03712 L/mol.arrow_forwardWhat volume of carbon dioxide (in L) will be produced from the complete combustion of 370.2 g of octane? Assume the carbon dioxide is at 27.67ºC and 0.872 atm. Give your answer to 3 sig figs. 2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(g)arrow_forward
- A10.12 mol sample of krypton gas is maintained in a 0.8044 L container at 301.1 K What is the pressure in atm calculated using the van der Waals' equation for Kr gas under these conditions? For Kr, a 2.318 L'atm/mol2 and b = 3.978x10-2 L/mol. atmarrow_forwardThe van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume V and temperature I for gases better than the Ideal Gas Law does: 72 (V-nb)= nRT The van der Waals equation of state. R stands for the gas constant and n for moles of gas. The parameters a and b must be determined for each gas from experimental data. Use the van der Waals equation to answer the questions in the table below. What are the units of a? What are the units of b? For argon the numerical value of a is 1.337 and the numerical value of b is 0.0320. Use the van der Waals equation to calculate the pressure of a sample of argon at -110.0 °C with a molar volume of 2.98 L/mol Round your answer to the correct number of significant digits. atm Use the Ideal Gas Law to calculate the pressure of the same sample under the same conditions. Round your answer to the correct number of significant digits. atm x10 §arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY