Question
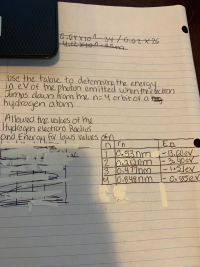
Transcribed Image Text:Use the talble to detemine the energy
in evof the photon emitted when thee bectran.
Jumps down from the n=4 orbit of a tasy
hydrogen atom
Allowed the values of the
Hydrogen electrons Raclius
and Energy fr lows values ofn
I En
10.53nm -13.60ev.
20,212nm |
3.40eV
3 0,477nm
M0.848nm -0.8ser
-l05lev
|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A 1eV photon’s wavelength is 120nm. Using ratio technique, calculate the wavelength of 5eV photon.arrow_forward1) The Sun cranks out about 4 x 1026 J/s, i.e. its radiant power pe = 4 x 10²6 W. For a square meter of area here on the Earth whose surface normal points directly to the Sun, how many watts are received? How many 550-nm photons would that correspond to? (The Earth-Sun distance is about 150x100 km.)arrow_forwardb) The visible spectrum of mercury is shown below: 200, 700 Mercury 280Hg 80¹ 623,4 [615,2 579 577 600 56 1546,1 (.. |502,5 ch 500 435,81 Explain what physical quantity is being measured on the scale and give the units. 407,8404,7 Explain the similarities and differences of the photons that give rise to the different bands on the spectrum. 1 400 R LAVENarrow_forward
arrow_back_ios
arrow_forward_ios