Question
thumb_up100%
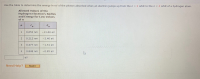
Transcribed Image Text:le to determine the energy in ev of the photon absorbed when an electron jumps up from the n = 2 orbit to the n = 4 orbit of a hydrogen atom.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A hydrogen atom is in its third excited state (n = 4). Using the Bohr theory of the atom, calculate the following. (a) the radius of the orbit nm (b) the linear momentum of the electron kg • m/s (c) the angular momentum of the electron J.S (d) the kinetic energy eV (e) the potential energy eV (f) the total energy eVarrow_forwardH-alpha line is a red visible spectral line in hydrogen atom with a wavelength of 656.3 nm. Consider five distant stars labeled A, B, C, D, and E. The light from these starts was detected on Earth and, after performing spectral analysis, the following H-alpha wavelengths were measured: AA = 667.5 nm, Ag = 650.4 nm, Ac = 653.5 nm, Ap = 660.3 nm, and AE = 664.9 nm. Which star has the slowest speed relative to Earth, in which direction and how fast does it move? The slowest star is? and it moves Select an answer The speed of the slowest star (in km/s), Vslowest = Which star has the fastest speed relative to Earth, in which direction and how fast does it move? The fastest star is? and it moves Select an answer Earth. The speed of the fastest star (in km/s), Vfastest Submit Question = Earth. Units Select an answer ✓ Units Select an answer ✓arrow_forwardFor an electron in a hydrogen atom, which of the following transitions would represent the largest quantum of energy being absorbed? Hydrogen Energy Transitions and Radiation Level n = ∞ n = 5 n = 4 486 nm n = 3 Infrared 434 nm 656 nm wavelengths n = 2 Visible wavelengths Ionization n = 1 Ultravioletarrow_forward
- An electron is excited from the n=1 ground state to the n=3 state in a hydrogen atom. Which of the following statements are true? Correct the false statements to make them true. (It may help to draw the Bohr model of the atom with the corresponding electron orbits.)a. It takes more energy to ionize (completely remove) the electron from n = 3 than from the ground state.b. The electron is farther from the nucleus on average in the n = 3 state than in the n = 1 state.c. The wavelength of light emitted if the electron drops from n = 3 to n = 2 will be shorter than the wavelength of light emitted if the electron falls from n = 3 to n = 1.d. The wavelength of light emitted when the electron returns to the ground state from n = 3 will be the same as the wavelength of light absorbed to go from n = 1 to n = 3.e. For n = 3, the electron is in the first excited state.arrow_forwardIn the picture are two diagrams of the first five orbits in the Bohr model labeled A - E. The dot in an orbit is the electron. The nucleus is not shown. Shown is a BEFORE and AFTER state of the atom. An energy level diagram for the Bohr hydrogen atom is shown. Which of these events has occurred in going from the BEFORE to the AFTER state? a. a photon of energy 0.97 eV has been emitted b. a photon of energy 1.51 eV has been absorbed c. a photon of energy 1.51 eV has been emitted d. an electron of energy 1.51 eV has been emitted e. a photon of energy 0.97 eV has been absorbedarrow_forwardご A 国 Using the Rydberg equation provided below determine the wavelength as well as the energy for a single photon emitted by the hydrogen atom's electron moving fromn 6 to n = 2. (more than one choice) %3D 1 = 1.10x10' m 1 1 where n>m 1 E=h c where h=6.63x10-3* J•s c=3.00 x10° m/sarrow_forward
- The Balmer series in hydrogen includes the Paschen series. has four lines in the ultraviolet. O includes both the Paschen series and the Lyman series. O has four lines in the visible. O includes the Lyman series.arrow_forwardA hydrogen atom is in its fourth excited state. The atom emits a 1.28E+3nm wavelength photon. Determine the maximum possible orbital angular momentum of the electron after emission. Express your answer as multiples of hbar.arrow_forwardA photon is emitted when a hydrogen atom undergoes a transition from the n = 6 state to the n = 2 state. Calculate values for the following. (a) the wavelength nm (b) the frequency Hz (c) the energy of the emitted photon eVarrow_forward
arrow_back_ios
arrow_forward_ios