
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Help
![Use the plot above to determine your answer. If functions I, II, and III have THE SAME angular
momentum quantum number (1), identify them.
V [ Choose ]
2p
1s
II
4d
3d
3s
III
2s
3p
%3D](https://content.bartleby.com/qna-images/question/67e70f86-06d8-4b86-a501-b94e483472a5/d3d4793d-5bf9-4ac2-8f1f-b36d35c7fb52/m6ik94h_thumbnail.png)
Transcribed Image Text:Use the plot above to determine your answer. If functions I, II, and III have THE SAME angular
momentum quantum number (1), identify them.
V [ Choose ]
2p
1s
II
4d
3d
3s
III
2s
3p
%3D
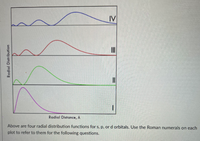
Transcribed Image Text:IV
Radial Distance, A
Above are four radial distribution functions for s, p, or d orbitals. Use the Roman numerals on each
plot to refer to them for the following questions.
Radial Distribution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ipboard Font Paragraph Styles Aspirin Yield Lab NAME: 6. How much aspirin is needed for Part B #1? PRE-LAB 1. What is an antipyretic? REPORT: 2. What is an analgesic? A. Preparation of Aspirin DAY 1 3. What is the "active ingredient" in aspirin? Why is it not ingested directly? 1. Mass of salicylic acid (g) 2. Mass of filter paper (g) 4. In this experiment, 2.00 g of salicylic acid reacts with an excess amount of acetic anhydride. Calculate the theoretical yield of acetylsalicylic acid for this synthesis. 3. Mass of dried filter paper plus sample (g) 4. Theoretical yield of aspirin (g) 5. Experimental (actual) yield of aspirin (g) 5. A0.331 g sample of aspirin prepared in the laboratory was dissolved in 95% ethanol and titrated to a phenolphthalein endpoint with 16.7 ml of 0.107 M NAOH. 7. Volume of water used in the experiment 8. Mass of aspirin dissolved in the experiment (see A7 a. Calculate the moles of acetylsalicylic acid in the aspirin sample. 6. Experimental yield, corrected…arrow_forwardHello can you please help me answer this?arrow_forwardhelp mearrow_forward
- Need help understanding how to do these problems. Can you show me step by step how to do a couple of these?arrow_forwardA doctor has ordered for a week 0.35 grams of Carvedilol for a patient with high blood pressure. If your stock on hand consists of 25 mg tablets, how many tablets will you need for 1 day's treatment for the patient? QUESTION 13 O a. 2 O b. 14 O C. 7 O d. 20 O e. 1 QUESTION 14arrow_forwardWhat would step 1 and 2 be?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY