
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Only typed answer otherwise leave it
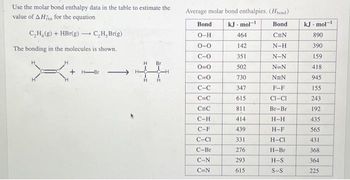
Transcribed Image Text:Use the molar bond enthalpy data in the table to estimate the
value of AH for the equation
C₂H₂(g) + HBr(g) →→ C,H, Br(g)
-
The bonding in the molecules is shown.
-Br
H Br
Average molar bond enthalpies. (Hbond)
Bond.
C=N
N-H
N-N
N=N
NEN
F-F
CI-CI
Br-Br
H-H
H-F
H-CI
H-Br
H-S
S-S
Bond
O-H
0-0
C-O
0=0
C=0
C-C
C=C
C=C
C-H
C-F
C-CI
C-Br
C-N
C=N
kJ.mol-1
464
142
351
502
730
347
615
811
414
439
331
276
293
615
kJ. mol-1
890
390
159
418
945
155
243
192
435
565
431
368
364
225
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- [References] Use the References to access important values if needed for this question. Taking logarithms and antilogarithms is necessary to solve many chemistry problems. For practice, complete the following table, where N is a number. log N 7.92 1.867 -1.402 Submit Answer Retry Entire Group 4 more group attempts remaining Previous Next Save and Exitarrow_forwardwhat is an intensive observation of gold?arrow_forwardPlease solve the question I recieved incorrect and show work!!arrow_forward
- Abdelra X M Mathway | Algebra Prol X G molar mass of zinc chl x Launch Meeting-Zoom x G 0.16kg to g-Google Se x+ www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lix5uFZIVj2iEJjQd1WoxT77ErtzZpGbzESWMk5SbMzyGfsmkwQSq2n2qsprma6JcCeltVTwZDkOLh51Rytx20V. O GASES, LIQUIDS, AND SOLIDS Using the Kf and Kb equations with electrolytes Jeneen v A certain liquid X has a normal boiling point of 93.40°C and a boiling point elevation constant K=1.46 °C-kg mol . A solution is prepared by dissolving -1 9. some iron(III) nitrate (Fe(NO,)) in 550. g of X. This solution boils at 93.8 °C. Calculate the mass of Fe(NO,) that was dissolved. Be sure your answer is rounded to the correct number of significiant diglts. x10 Check Explanation 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibility M9arrow_forwardDensity problem to find the grams or mass.arrow_forwardgive electronic configuration of any one of coinage metal.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY