
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:* 60%
iPad
2:23 PM
+
openvellum.ecollege.com
Search Textbook Solutions | Chegg.com
61%iPad2:20 PM+openvellum.ecollege.comSearch...
Course Home
My Courses
KOnline homework for CH08
Course Home
Chapter 8 Multiple-Choice Problem 45
>
35 of 41
Syllabus
Scores
Review | Constants
I Periodic Table
еТext
Document Sharing
User Settings
Part A
Course Tools
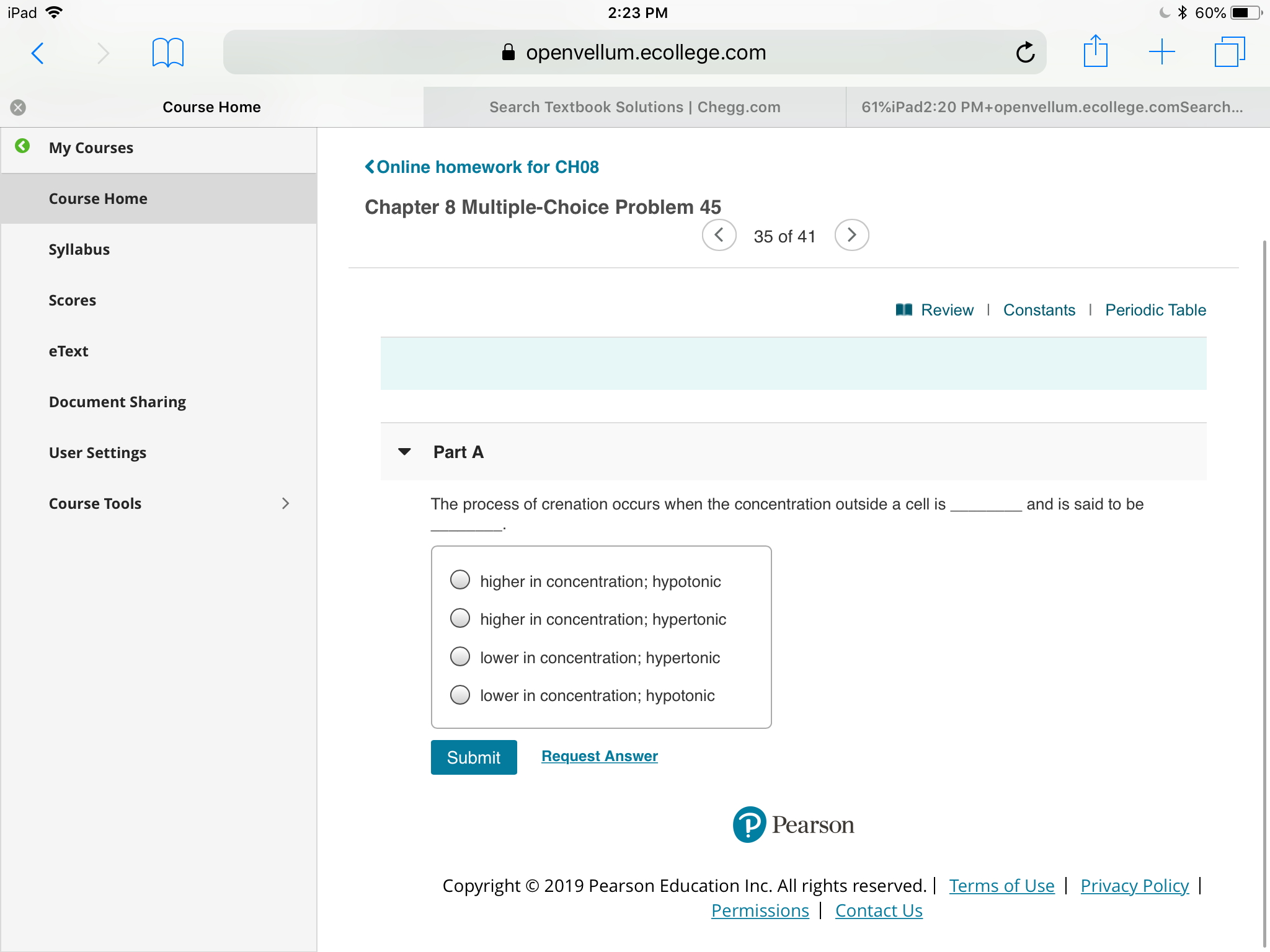
The process of crenation occurs when the concentration outside a cell is
and is said to be
higher in concentration; hypotonic
higher in concentration; hypertonic
lower in concentration; hypertonic
lower in concentration; hypotonic
Request Answer
Submit
Pearson
Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy Policy
Permissions Contact Us
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 00 %2A # 3. 5o09 awww.awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lijulplKKWv4bwenmyrLYoBHBAAsJtmzj53GErnx_viTdHblGMF9jvgkSu_nrizREmdQXHPJbMzqOmOrXJcuonZITOhB66q?1oBw7QYlbav. LEKS-Courtney Wh New Tab + x O CHEMICAL REACTIONS Percent yield of chemical reactions Courtney S0 OC Gaseous butane CH. reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). If 2.32 g of water is produced from the reaction of 1.74 g of butane and 10.2 g of oxygen gas, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it. % 固 Explanation Check ©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility 24 %23 2 4. 9. 5. 8. by k 4 69 C. alt 123arrow_forwardProject PART I. Our Carbon Budget A major driver of global climate change is the emissions of greenhouse gases such as CO₂ into the atmosphere from human activities such as the burning of fossil fuels for transportation, electric power generation, manufacturing, agriculture, home heating, and so forth. Global average temper- atures are now slightly higher than 1° C above their pre-Industrial Revolution levels. Damaging sea level rise and extreme weather events (such as the recent wildfires in Canada that led to the unhealthy air in NYC last summer) will become an increasingly major problem around the world if global average temperature rises more than 1.5° C. Scientists estimate that the total additional amount of CO₂ that can be put into the atmosphere without reaching the 1.5° C level is about 51 parts per million (ppm) or approximately 400 Gigatons (Gt). Let's call 51 ppm our carbon budget. In this project, we want to use linear systems of differential equations to analyze the…arrow_forwardMr. Nugent (he/him|they/th... Diana Nguyen 2 jocelyn p Chem Classwork for Che X Balancing Eqnser Presentation Sess X Counting At 0ogle.com/presentation/d/1auph8DuajnWJDcUF7B02ieDMk6NBNvvXseY.. okmarks Links Attendance Drives A Classroom M Gmail Phys Morning Chem Morn ce the chemical equation: As + Na AsO, + _ H, NaOH →arrow_forward
- Mla pnc.com/women Mail - Whi x O Grades for x Course Ho X G oxide cont X G Exercise 4.x E 4 - Molecu x O Bananas a X 1.84 x 10- x + envellum.ecollege.com/course.html?courseld 16985674&OpenVellumHMAC=a0b841caaee7feddab1623a6c83a92c0#10001 Review | Constants I Periodic Table Percent composition refers to the mass percent of each element in a compound: mass of element Part A mass percent = x 100% mass of compound > For example, the percent composition of water, H2O. is 11.2% hydrogen and 88.8% oxygen. Therefore, a 100-g sample of water contains 11.2 g of hydrogen atoms and 88.8 g of oxygen atoms. A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composition (by mass) of the following hydrocarbon: CH22 Enter the percentages of carbon and hydrogen numerically to four significant figures, separated by commas. The periodic table will be useful when doing this problem. You can access a periodic table by clicking the "Tools" link in the upper right…arrow_forwardSolving part B and C E°cell from part A = 2.33Varrow_forwardof Matter Tut x C Clever | Portal e States of Matter: Mästery Test A fl.app.edmentum.com/assessments-delivery/ua/mt/launch/49105571/838227860/AHR0CHM Next O States of Matter: Mastery Test 1 Select the correct answer. What changes occur at the molecular level when butter melts in a hot pan? the motion of the butter molecules remalns the same O A. O B. the motion of the butter molecules Increases O C. the motion of the butter molecules decreases O D. the motion of the butter molecules stops completely Reset Next rights reserved.arrow_forward
- 2 3 4 -5 g . Use this information to calculate K.n for PbCO2. The solubility of PbCO, in water at 25 °C is measured to be 7.3 × 10 sp Round your answer to 2 significant digits. Continue O 2022 McGraw Hill LLC. All Rights Reserved. Ter II 20arrow_forwardi need help solving for number 4arrow_forwardxMail - Mary x D2L Grades - Sp X G when does X AT HOMEWORX Id=5735112480241329813180832311&elSBN=9781305862883&id=1707785907&snapshot!... Back References Use the References to access important values if needed for this question. grams oxygen Submit Answer MindTap - C X 2. Conservation of Mass: Macroscopic: This is group attempt 2 of 10 Under certain conditions, the substance mercury(II) oxide can be broken down to form mercury and oxygen. G If 27.8 grams of mercury(II) oxide react to form 25.7 grams of mercury, how many grams of oxygen must simultaneously be formed? Autosaved at 8:38 PM G Which of th X Q Search this Darrow_forward
- Fill in the missing coefficientsarrow_forward100% 41 Safari File Edit View History Bookmarks Window Help Wed 4:51 PM A uk.instructure.com My Questions | bartleby Course Details University of Kentucky - CHE 111 Lab - Fall19 - FRENCH: Quiz.. Calculate the Molarity of Sodium Hydroxide? | Yahoo Answers = CHE111-017-032 > CHE111 Sections 017 to 32 (Tuesdays): General Chemistry I Laboratory (Fall 2019) Fall 2019 Home Account Announcements Dashboard Question #: 11 Syllabus Course Details An aqueous solution containing 0.3845 g of KHP (KHC;H4O4) was titrated with a solution of sodium hydroxide, producing the following titration curve. What is the concentration of the NaOH solution? Courses Modules Titration of KHP with NaOH Piazza Groups 14 Grades 12 Calendar 10 Inbox 6. Help Volume NaOH (ml) DEC 18arrow_forwardOWLV2 | Online teaching and X 0mySigTau xb Login | bartleby X M COMM.1113: FUND OF ORA X G what food has c - Google Se X + om/ilrm/takeAssignment/takeCovalentActivity.do?locator=assignment-take D [References] hotogray lenses incorporate small amounts of silver chloride in the glass of the lens. When light hits the AgCl particles, the following reaction occurs: hv AgCl Ag + Cl The silver metal that is formed causes the lenses to darken. The enthalpy change for this reaction is 2.90 × 10² kJ/mol. Assuming all this energy must be supplied by light, what is the maximum vavelength of light that can cause this reaction? Wavelength %3D nm Submit Answer Try Another Version 3 item attempts remaining Previous Nextarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY