
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
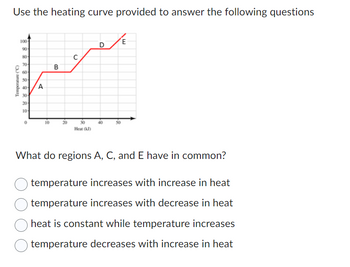
Transcribed Image Text:Use the heating curve provided to answer the following questions
Temperature (°C)
100-
90-
80-
70-
60
50-
40- A
30-
20-
10-
0
10
B
C
Heat (kJ)
D
40
E
What do regions A, C, and E have in common?
temperature increases with increase in heat
temperature increases with decrease in heat
heat is constant while temperature increases
temperature decreases with increase in heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 45-g aluminum spoon (specific heat 0.88 J/g C) at 24 C is placed in 180 mL (180 g) of coffee at 85 C and the temperature of the two become equal. (a) What is the final temperature when the two become equal? Assume that coffee has the same specific heat as water. (b) The first time a student solved this problem she got an answer of 88 C. Explain why this is clearly an incorrect answer.arrow_forwardA 110.-g sample of copper (specific heat capacity = 0.20 J/C g) is heated to 82.4C and then placed in a container of water at 22.3C. The final temperature of the water and copper is 24.9C. What is the mass of the water in the container, assuming that all the heat lost by the copper is gained by the water?arrow_forwardCopper is used in building the integrated circuits, chips, and printed circuit boards for computers. When 228 J of heat are absorbed by 125 g of copper at 22.38C, the temperature rises to 27.12C. What is the specific heat of copper?arrow_forward
- The BTU (British thermal unit) is the unit of energy most commonly used in the United States. One joule=9.48104 BTU. What is the specific heat of water in BTU/lbF? (Specific heat of water is 4.18 J/g C.)arrow_forwardYou have two samples of different metals, metal A and metal B, each having the same mass. You heat both metals to 95C and then place each one into separate beakers containing the same quantity of water at 25C. a You measure the temperatures of the water in the two beakers when each metal has cooled by 10C and find that the temperature of the water with metal A is higher than the temperature of the water with metal B. Which metal has the greater specific heat? Explain. b After waiting a period of time, the temperature of the water in each beaker rises to a maximum value. In which beaker does the water temperature rise to the higher value, the one with metal A or the one with metal B? Explain.arrow_forwardSwimming Pool A swimming pool measuring 20.0m12.5m is filled with water to a depth of 3.75m. If the initial temperature is 18.4°C, how much heatmust be added to the water to raise its temperature to29.0°C? Assume that the density of water is 1.000 g/mL.arrow_forward
- Which produces more heat? Os(s)2O2(g)OsO4(s)orOs(s)2O2(g)OsO4(g) for the phase change OsO4(s)OsO4(g)H=56.4kJarrow_forwardThe “Chemistry in Focus” segment Nature Has Hot Plants discusses thermogenic, or heat-producing, plants. For some plants, enough heat is generated to increase the temperature of the blossom by 15 °C. About how much heat is required to increase the temperature of 1 L of water by 15 °C?arrow_forwardIf 125 J of heat energy is applied to a block of silver weighing 29.3 g, by how many degrees will the temperature of the silver increase? (See Table 10.1.)arrow_forward
- A 500-mL bottle of water at room temperature and a 2-L bottle of water at the same temperature were placed in a refrigerator. After 30 minutes, the 500-mL bottle of water had cooled to the temperature of the refrigerator. An hour later, the 2-L of water had cooled to the same temperature. When asked which sample of water lost the most heat, one student replied that both bottles lost the same amount of heat because they started at the same temperature and finished at the same temperature. A second student thought that the 2-L bottle of water lost more heat because there was more water. A third student believed that the 500-mL bottle of water lost more heat because it cooled more quickly. A fourth student thought that it was not possible to tell because we do not know the initial temperature and the final temperature of the water. Indicate which of these answers is correct and describe the error in each of the other answers.arrow_forwardA block of aluminum and a block of iron, both having the same mass, are removed from a freezer and placed outside on a warm day. When the same quantity of heat has flowed into each block, which block will be warmer? Assume that neither block has yet reached the outside temperature. (See Table 6.1 for the specific heats of the metals.)arrow_forwardHow much heat, in joules and in calories, is required to heat a 28.4-g (1-oz) ice cube from 23.0 C to 1.0 C?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co