
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
How can we answer these questions based on the diagrams ?
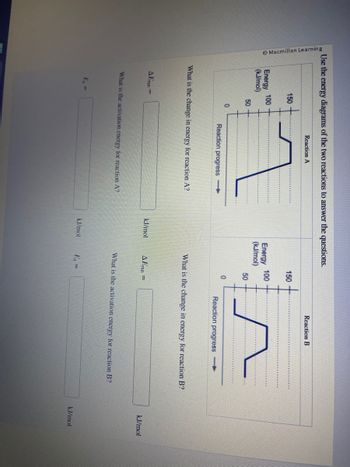
Transcribed Image Text:Use the energy diagrams of the two reactions to answer the questions.
Macmillan Learning
150
Energy 100
(kJ/mol)
AErxn=
50
E₁ =
0
Reaction A
What is the change in energy for reaction A?
Reaction progress-
What is the activation energy for reaction A?
kJ/mol
kJ/mol
150
Energy 100
(kJ/mol)
50
0
E₁ =
AErxn =
Reaction B
What is the change in energy for reaction B?
Reaction progress-
What is the activation energy for reaction B?
kJ/mol
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student conducts an experiment using an unknown hydrate and obtains the following data Crucible 24.31g Crucible + hydrate 29.31g Crucible + anhydrate 28.26g Further experimentation demonstrates the anhydrate is composed of 29.44% magnesium, 23.55% sulfur and 47.01% oxygen by mass. A. Determine the mass of hydrate, anhydrate and water found in the sample. B. Determine the emperical formula for the anhydrate. C. Determine the formula for the hydrate.arrow_forwardAre students allowed to place a hot crucible on the balance? (Choose all correct answers) Yes. Hot objects do not harm the balance. No. Weighing hot objects will result in an incorrect mass. No. Weighing hot objects could damage the balance pan, balance, or both. Yes. Hot objects on the balance have no effect on the mass.arrow_forwardA student measured the temperature of boiling water to be 105.25 C. the correct temperature for boiling water is 100 C at 1 atm. What went wrong with the experimentarrow_forward
- Discuss the various ways in which chemistry has influenced the art world, a. including the development of new materials, b. the study of chemical reactions, c.and the use of chemistry in conservation and restoration efforts.arrow_forwardWhich of the following is a pure substance that can be broken down into simpler substances by chemical means? Solution Compound Heterogeneous mixturearrow_forwardAlbert Einstein is quoted as saying, “Not only to know how nature is and how her transactions are carried through, but also to reach as far as possible the utopian and seemingly arrogant aim of knowing why nature is thus and not otherwise.” What do you think Einstein meant by this?arrow_forward
- How many kcal/mol are in 271.86 kJ/mol? Do not put units in your answer. Give your answer to two decimal places.arrow_forwardHöhörs Chemistry- 4th Hour - Dr. Paul / Gases/Lesson 148 What does the kinetic molecular theory tell us about the temperature of a substance? O The temperature of a substance does not affect the motion of the particles. OA substance with a lower temperature has particles that move faster. O A substance with a higher temperature has particles that move slower. O A substance with a higher temperature has particles that move faster.arrow_forwardEvery year Every second (1 year 365 days).arrow_forward
- 25.3 g of magnesium reacts with 44.3 g of copper (ll) nitrate to form copper and magnesium nitrate. What mass of copper will form?arrow_forwardO MEASUREMENT Adding or subtracting and multiplying or dividing measurements A chemistry student must write down in her lab notebook the concentration of a solution of sodium hydroxide. The concentration of a solution equals the mass of what's dissolved divided by the total volume of the solution. Here's how the student prepared the solution: • The label on the graduated cylinder says: empty weight: 1.5 g • She put some solid sodium hydroxide into the graduated cylinder and weighed it. With the sodium hydroxide added, the cylinder weighed 36.669 g. • She added water to the graduated cylinder and dissolved the sodium hydroxide completely. Then she read the total volume of the solution from the markings on the graduated cylinder. The total volume of the solution was 37.11 mL. What concentration should the student write down in her lab notebook? Be sure your answer has the correct number of significant digits. g mL Explanation -1 Check 99+ 0/5 VUDU © 2023 McGraw Hill LLC. All Rights…arrow_forwardIn a lab students tested two properties of four substances. The results of the tests are shown the table below. Substance Phase at room Reaction with Reaction to flame temperature water Hydrogen Colorless Gas None Explodes Potassium Silver Solid Bubbling and reaction is hot. Burns and is likely to explode Sulfur Yellow Solid None Burns slowly and turns a color Strontium chloride White Crystalline Solid Dissolves Melts Which substance and explanation proves that no chemical change took place? O A. Hydrogen because it did not react with water and will explode when exposed to a flame. O B. Potassium because it bubbles in water and will explode when exposed to a flame Type here to searcharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY