
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
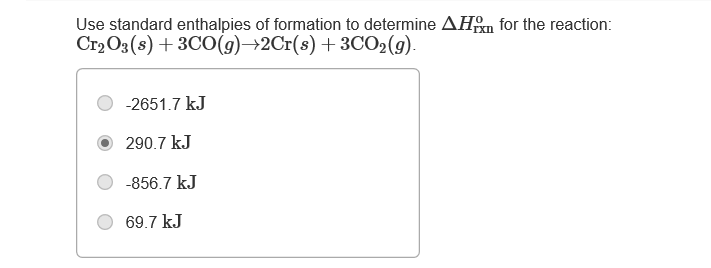
Transcribed Image Text:Use standard enthalpies of formation to determine AHm for the reaction:
Cr2O3(s) + 3CO(9)→2Cr(s) +3CO2(g).
-2651.7 kJ
290.7 kJ
-856.7 kJ
69.7 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 26. Calculate the enthalpy change for the reaction below. NO(g) + O(g) →→→ NO₂(g) Given following data: NO(g) + O₂(g) →→ NO₂(g) + O₂(g) 03(g) → 1.50₂(g) 0₂(g) →20(g) AH-198.9 kJ AH = -142.3 kJ ΔΗ = 495.0 kJarrow_forwardGiven the overall reaction from the previous problem: 2 CO (g) + 6 H2 (g) D 2 CH4 (g) + 2 H2O (g) Using Le Chatelier’s principle, predict which direction the reaction will shift to reestablish equilibrium for each of the following changes: Calculate the enthalpy of reaction for this reaction using the standard thermodynamics values for the reactants and products. Is the reaction endothermic or exothermic? Now, use Le Chatelier’s principle again to determine the direction the equilibrium will shift if heat is added to the reaction. If 35 kPa of CO is mixed with 35 kPa of hydrogen in a 1.5 L container at 25°C, what is the theoretical yield of methane?arrow_forwardGiven that enthalpy of formation of the several compounds: C(s)+O2(g)>CO2(g);AH=-393.5kJ S(s)+O2(g)→SO2(g);AH=-296.8kJ C(s)+2S(s)→CS2(1);AH=+87.9 kJ Determine the enthalpy of combustion reaction of carbon disulfide!arrow_forward
- Using the provided enthalpy of formation, determine the enthalpy for the reaction: 3A (s)+ 2B (I) ->C (I) + 4D(s) substance AH;° (KJ/mol) A (s) -286arrow_forwardCalculate the enthalpy of reaction for the following reaction: Fe₂O3(s) + 3CO(g) → 2Fe(s) + 3CO₂(g) AH,(Fe₂O3(s)) = -824.2 kJ/mol AH(CO(g)) = -110.5 AH,(Fe(s)) = ? AH(CO₂(g)) = -393.5 kJ/mol Answer: How much energy in kJ is required to heat a calorimeter whose heat capacity is 113.4 J/°C from 23.5°C to 55.0°C?arrow_forwardA 3.000 g sample of food was then burned in a calorimeter to determine its Calories per gram content. 50mL of water were used to determine ΔT of the reaction. Initial temperature of the water at the start of the combustion reaction was 20oC, final temperature was 99oC. Calorimeter constant for this calorimeter is 10.0J/oC. How much heat has been absorbed by the water sample?arrow_forward
- 16. Use enthalpies of formation given to determine the standard enthalpy of reaction for the following: ed oioege Al2O3 (s) +2 Fe 2 Al (s) + Fe2O3 (s) AH° =??? > AH°r(kJ/mol) -825.5 -1676 17. Use the enthalpies of formation and the enthalpy of reaction given below to determine the enthalpy of formation for solid CaC2. CaC2 (s) + 2 H2O (1) → Ca(OH)2 (s) + C2H2 (g) AH° =-127 kJ AH° (kJ/mol) ??? -286 -986 +227arrow_forwardAluminum metal can be produced by reducing solid aluminum oxide (Al2O3) with carbon (graphite). Carbon monoxide is the other product. What is the enthalpy change (AH° in kJ) when 25.0 g of aluminum metal is produced in this manner? You will need to balance the chemical equation. Enthalpies of formation (in kJ mol¯¹): Al2O3(s) -1675.7 CO(g) -110.5 Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a -623 b 623 с -734 d 734arrow_forward2. Please calculate the standard enthalpy of reaction (AHrxn, in kJ/mol) for the preparation of nitrous acid, HNO2, using the thermodynamic data given below. Preparation of nitrous acid: HCl(g) + NaNO2(s) → HNO2(1) + NaCl(s) 2NaCl(s) + H₂O(l) → 2HCl(g) + Na₂O(s) NO(g) + NO₂(g) + Na2O(s) → 2NaNO2 (s) NO(g) + NO₂(g) → N₂O(g) + O₂(g) 2HNO2(1)→ N₂O(g) + O2(g) + H₂O(g) H₂O(1)→ H₂O(g) ΔΗ = 507.31 kJ/mol AH = -427.14 kJ/mol AH = - 42.68 kJ/mol 34.35 kJ/mol ΔΗ ΔΗ = 44.012 kJ/mol =arrow_forward
- What is the enthalpy of reaction, ΔHrxn for the reaction of nitrogen gas with oxygen gas to produce NO2(g), based on the following information? These reactions are not at standard state or at 298 K. N2(g) + O2(g) → 2 NO(g); ΔH = 332.9 kJ2 NO2(g) → 2 NO(g) + O2(g); ΔH = 718.4 kJ Report your answer in kJ to 1 decimal place.arrow_forwardGiven the following reactions Fe2O3 (s) + 3CO (s) → 2Fe (s) + 3CO2 (g) ΔH = –28.0 kJ mol–1 3Fe (s) + 4CO2(s) → 4CO (g) + Fe3O4(s) ΔH = +12.5 kJ mol–1 Determine the enthalpy of the following reaction in kJ mol–1 3Fe2O3 (s) + CO (g) → CO2 (g) + 2Fe3O4 (s) Select one: a. 40.5 b. +109 c. –109 d. –59.0 e. –15.5arrow_forward7arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY