
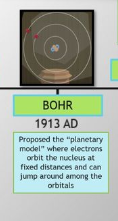
Hints- A model of the atom which explained the atomic emission spectrum of hydrogen was proposed by Neils Bohr. Bohr suggested that an atomic spectrum is created when the electrons in an atom move between energy levels. In
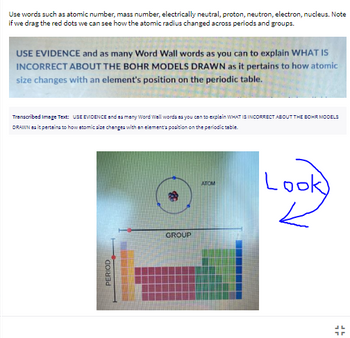
The arrow is pointing at the model you are supposed to look at.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

- Why was Bohr's theory for the hydrogen atom initially accepted, and why was it ultimately discarded?arrow_forwardHow are the Bohr model and the Rutherford model of the atom similar? How are they different?arrow_forward6.106 When Bohr devised his model for the atom, was he using deductive or inductive reasoning? Explain your answer.arrow_forward
- How many sublevels are there in an atom with n=4?arrow_forwardAccording to Bohr, the electron in the hydrogen atom moved around the nucleus in circular paths called .arrow_forwardAccording to the Bohr theory, which of the following would have the higher energy? a. An electron in an orbit close to the nucleus b. An electron in an orbit located farther from the nucleusarrow_forward
- In J. J. Thompson’s experiment depicted in Figures 1.10 and 1.11, assume that the electrons are initially traveling at a speed of 8.00106ms1 in the +x direction and the electrostatic plates have a length of l=0.10m , between which the electrons are deflected toward the -y direction (downward) by an electric field of magnitude 200Vm1 . (Note that 1volt=1V=1JC1 .) Calculate S, the magnitude of the downward displacement, if the fluorescent screen is located 0.90 m beyond the exit of the region between the two electrostatic plates. No magnetic field is applied to counteract the electrostatic force. (Caution: L is not 1.0 m nor 0.9 m; see Figure 1.11.)arrow_forwardHow are the Bohr model and the quantum mechanical model of the hydrogen atom similar? How are they different?arrow_forwardState the postulates of quantum mechanics introduced throughout the chapter in your own words.arrow_forward
- Describe the activity of electrons according to the planetary model of the atom that appeared after the Rutherford scattering experiment.arrow_forwardIn the Bohr model of the hydrogen atom, the electron occupies distinct energy states. In one transition between energy states, an electron moves from n=1 to n=2. Is energy absorbed or emitted in the process? Does the electron move closer to or farther from the nucleus?arrow_forwardWhich statement is true of the quantum mechanical model, but not of the Bohr model? a. Electrons orbit the nucleus in simple circular orbits, just like planets orbit the Sun. b. The exact path that an electron follows within an atom cannot be specified. c. The electron is attracted to the nucleus of the atom.arrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHERChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





