
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
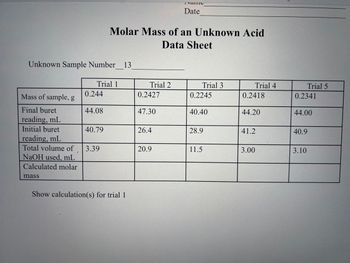
Transcribed Image Text:Calculated
molarity
Standardization of Sodium Hydroxide
Data Sheet
Trial 1
Mass of KHP, g
Final buret
reading, mL
Initial buret
reading, mL
Total volume of 14.80
NaOH used, mL
0.4961
34.20
49.00
Trial 2
0.5028
23.40
39.50
16.10
Name
Date
Show the calculation for the molarity of trial 1
Trial 3
0.4987
37.5
23.2
14.30
Trial 4
0.4954
41.90
30.3
11.60
Trial 5
0.4918
45.10
26.12
18.98
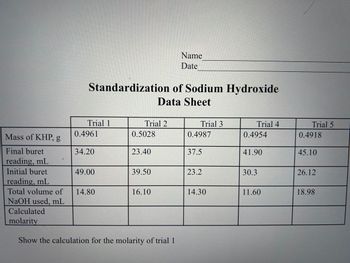
Transcribed Image Text:Calculated
molarity
Standardization of Sodium Hydroxide
Data Sheet
Trial 1
Mass of KHP, g
Final buret
reading, mL
Initial buret
reading, mL
Total volume of 14.80
NaOH used, mL
0.4961
34.20
49.00
Trial 2
0.5028
23.40
39.50
16.10
Name
Date
Show the calculation for the molarity of trial 1
Trial 3
0.4987
37.5
23.2
14.30
Trial 4
0.4954
41.90
30.3
11.60
Trial 5
0.4918
45.10
26.12
18.98
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Remaining Time: 1 hour, 01 minute, 47 seconds. * Question Completion Status: QUESTION 2 The number of water molecules in 2.3 mg of water is O 6.02 x 1023 O 77.0 x 1023 O 7.7 x 1023 O 7.7 x 1019 none of the above QUESTION 3 A ctudent makes a colution bu discolvina 254a ofNDOH int0 450 gof water Wh. Click Save and Submit to save and submit. Click Save All Answers to save all answer ere to search DELLarrow_forwardm/course.html?courseld=16481757&OpenVellumHMAC=feeaec83fa5cc7b2f32454414caf19a1#10001 Part C In the gaseous state, chlorine exists as a diatomic molecule Cl2 (Molar mass = 70.9 g/mol). Calculate the number of moles of chlorine present in 140 g of chlorine gas. Express the quantity in moles to three significant figures. • View Available Hint(s) ? mol Moles of chlorine gas = Submit Previous Answers X Incorrect; Try Again Multi-step problems A problem that asked you to convert molecules to grams could require two steps 1. convert the molecules to moles P Pearson 2convert to moles to areme Copyright O 2021 Pearson Education Inc. All rights reserved. I Terms of Use | Privacy Policy I Permissions | Contact Us |arrow_forwardO 0.970 M QUESTION 37 A hospital saline solution is analyzed to confirm its concentration. A 50.0 mL sample with a mass of 50.100 g is evaporated to dryness. The solid sodium chloride residue has a mass of 0.656 g. What is the mass/mass percentage? O 2.51 % O 1.31 % O 13.1% O 3.77% QUESTION 38 A hosnital salinıarrow_forward
- Empirical Formula for Unknown Sample Data1. Mass of crucible 28.072(grams)2. Mass of crucible + Unknown 30.528(grams)3. Mass of crucible + Na + S 29.782(grams)4. Mass of crucible + Na 28.786(grams)5. Mass of Na 0.714 6. Mass of S 0.996 7. Mass of O 0.7468. Mass of Unknown Sample 2.51 using the information above find the moles for the following elements: Moles of Na Moles of S Moles of Oarrow_forwardConc. of NaOH: TRIAL 1 TRIAL 2 TRIAL 3 Mass of flask 81.061 69.692 96.039 Mass of flask and vinegar 83.010 71.578 97.940 Mass of vinegar 1.949 1.886 1.901 Volume of vinegar 2.00 2.00 2.00 Initial buret reading Final buret reading 17.0 17.5 16.0arrow_forwardVolume (mL) Trial 1 Trial 2 Trial 3 Final volume (mL) 24.35 47.60 20.60 Initial volume (mL) 1.25 25.15 42.85 Volume Added 23.10 22.45 22.35 Average Volume Added 22.63 mL Concentration of NaOH 0.9971 M % Acetic Acid in Vinegar Assuming all the acid to be acetic, calculate the number of grams of acid per 100 mL of vinegar solution. Assuming that the density of vinegar is 1.000, what is the percentage of acetic acid by weight in vinegar? Average your results in the usual manner. % of Acetic Acid = (mL of NaOH added)(concentration of NaOh)/mL of vinegar samplearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY