
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How would i solve
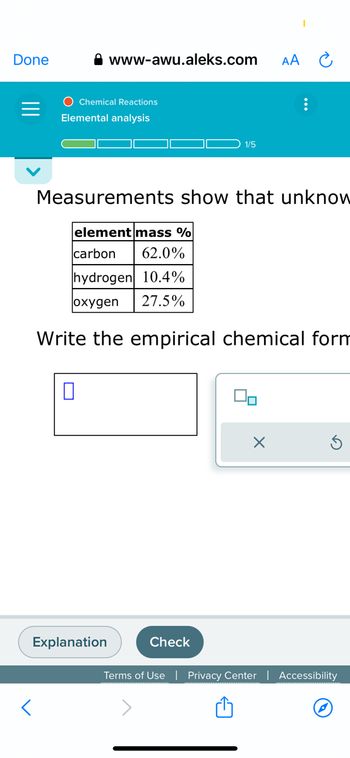
Transcribed Image Text:The webpage from https://www.awu.aleks.com features an educational tool on chemical reactions focusing on elemental analysis. The page displays progress labeled as "1/5" steps toward completing a task.
### Elemental Analysis
#### Measurements show that unknown substance consists of:
| Element | Mass % |
|-----------|--------|
| Carbon | 62.0% |
| Hydrogen | 10.4% |
| Oxygen | 27.5% |
Below the table, a prompt asks:
"Write the empirical chemical formula."
**Input Box**: There is a blank input box provided for user input, along with options to submit or reset the response.
At the bottom, functional buttons are labeled "Explanation" and "Check" to assist users in verifying or understanding their answers.
**Note**: Empirical formulas represent the simplest whole-number ratio of elements in a compound, derived from the given percentage composition.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What does it mean to say an equation is balanced? Why is it important for an equation to be balanced?arrow_forwardNitrogen (N2) and hydrogen (H2)react to form ammonia (NH3). Consider the mixture of N2 and H2 in a closed container as illustrated below: Assuming the reaction goes to completion, draw a representation of the product mixture. Explain how you arrived at this representation.arrow_forwardWhat is the theoretical yield for a reaction, and how does this quantity depend on the limiting reactant?arrow_forward
- Many cereals are made with high moisture content so that the cereal can be formed into various shapes before it is dried. A cereal product containing 58% H2O by mass is produced at the rate of 1000. kg/h. What mass of water must be evaporated per hour if the final product contains only 20.% water?arrow_forwardExplain how an equation can be balanced even if the number of reactant particles differs from the number of product particles.arrow_forwardWrite the balanced chemical equation for the complete combustion of adipic acid, an organic acid containing 49.31% C, 6.90% H, and the remainder O, by mass.arrow_forward
- Many cereals are made with high moisture content so that the cereal can be formed into various shapes before it is dried. A cereal product containing 58% H2O by mass is produced at the rate of 1000. kg/h. What mass of water must be evaporated per hour if the final product contains only 20.% water?arrow_forwardFor the chemical reaction C3H8O2+4O23CO2+4H2O how many product molecules are formed when nine C3H8O2 molecules react?arrow_forwardA common method for analyzing for the nickel content of a sample is to use a precipitation reaction. Adding the organic compound dimethylgly-oxime to a solution containing Ni2+ ions precipitates a red solid. Derive the empirical formula for the red solid based on the following composition: Ni, 20.315%; C, 33.258%; H, 4.884%; 0, 22.151%; and N, 19.392%.arrow_forward
- Calculate the amounts of reactants needed in a chemical reaction to produce a specified amount of product.arrow_forwardCalcium carbonate forms carbon dioxide and calcium oxide when it is heated above 900 °C in a limekiln. When heated to 1000 °C in a laboratory, 4.31 g calcium carbonate produces 2.40 g calcium oxide and 1.90 g carbon dioxide. Outline a method similar to combustion analysis by which you could determine the empirical formula for calcium carbonate from these data. Carry out the determination.arrow_forwardUsing solid circles for H atoms and open circles for O atoms, make a drawing that shows the molecular level representation for the balanced equation of H2 and O2 reacting to form H2O.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning