
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
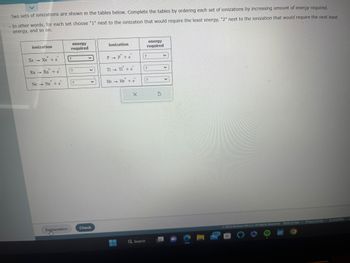
Transcribed Image Text:Two sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required.
In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least
energy, and so on.
ionization
Xe - Xẻ tè
1
Ra Ra + e
Ne Ne + e
Explanation
energy
required
?
?
?
v
v
y
Check
ionization
P→ P+e
T1 - TI + e
He He + e
1
X
?
Q Search
energy
required
?
?
S
V
V
v
99+
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
21
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This not a graded questionarrow_forwardWhat is the neutral atom that has its first two energy levels filled, has 1 electron in its third energy level, and has no other electrons? Enter the name of the element, not the abbreviation.arrow_forwardUsing the graph of successive ionization energies what element could the graph represent? Al Si CI Ionization Energy (kJ/mol) 35000 30000 25000 20000 15000 10000 5000 0 IE1 IE2 Successive lonization Energies IE3 IE4 IES IE6 IE7 Briefly explain how you knew which element the graph represented? Make sure to discuss core and valence electrons and relative energies. B I U X2 X2 IE8arrow_forward
- For each element determine the charge of the ion that will most likely form based on the data? What do you look for? Why does this happen? Based on the data, locate the family/column number for each element on the periodic table.arrow_forwardTwo sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. S-> S+ + e- Ca-> Ca+ + e- He-> He+ + e- --------------- K-> K+ + e- Fr-> Fr+ + e- Si-> Si+ + e-arrow_forward6arrow_forward
- Two sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. energy energy required ionization ionization required Fr Fr + e Kr Kr + e S - S + e ? Bi Bi + e ? Se - Se + e ? Ar → Ar + e ? > |>arrow_forwardTwo sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. 70°F Mostly cloudy ionization CI- CI+ e Rb Rb + e Ca Ca + e Explanation energy required ? ? ? Check v v ionization S Ste Bi Bie Fr Fre X Search energy required ? ? ? S v v V 4 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilityarrow_forwardWhat would you predict the charge of an ion from column 1A (or column 1 from ptable) to be? O a 2- O b 1- Oc 1+ Od 2+arrow_forward
- Rank the following elements by electron affinity, from most positive to most negative EA value. Iodine, Neon, Sulfer, Arsenic, Sodium.arrow_forwardTwo sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. energy energy ionization ionization required required olo + Sn с - с+е » Sn + e Ar Не — Не +e ? Cs Cs + e As - As +e ? Ga Ga + e ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY