
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
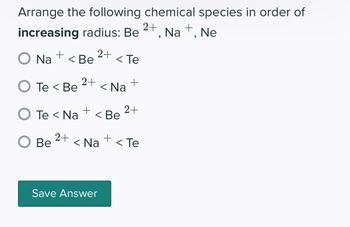
Transcribed Image Text:Arrange the following chemical species in order of
increasing radius: Be
Na +, Ne
O Na +
< Be
O Be
2+
2+
O Te< Be < Na
+
O Te < Na < Be
2+
< Te
Save Answer
+
2+
+
< Na <Te
2+
"
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What would you predict the charge of an ion from column 1A (or column 1 from ptable) to be? O a 2- O b 1- Oc 1+ Od 2+arrow_forwardArrange the following atoms in the order of increasing ionization energy: CI, S, Ar, Ne You may need to use the periodic table below. 1A 7A 8A Metals 3A 4A 5A 6A H He B C NO F Ne H 2A Metalloids Li Be Nonmetals Na Mg зв 4в 58 6B 7B — 88 — 1в 28 Al si P S CI Ar K Ca Sc Tiv Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Db Lanthanides Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Actinides Th Pa u Np Pu Am Cm Bk Cf Es Fm Md No Lr Increasing ionization energy Drag and drop your selection from the following list to complete the answer: Ne CI Ar Sarrow_forwardArrange the following in order of decreasing atomic radius: Al, Mg, Na, O O > Na > Mg > Al O Al > Mg > O > Na O > Al > Mg > Na O Al > Mg > Na > O Na > Mg > Al > Oarrow_forward
- Og is the noble gas after Rn. To go from [Rn] to [Og], you must fill four subshells (s, p, d, and f) with a total of 32 electrons. Thus, the atomic numbers of sixth and seventh period elements of the same group differ by 32. To go from [Og] to the next noble gas, however, you would theoretically fill five subshells (s, p, d, f, and g). How many electrons are needed to fill all five subshells? number of electrons: Element 106 in the periodic table is Sg. Determine the atomic number of the element just below Sg in the periodic table. atomic number:arrow_forwardWhich element would have the greatest ionization energy? S, Ge, Al, K, or Sr Which equation below illustrates the first ionization energy for strontium? A, B, C, D, E, or F A) Sr (g) + 2e– → Sr–2 (g) B) Sr (g) → Sr+2 (g) + 2e– C) Sr+ (g) →Sr+2(g) + e– D) Sr (g) → Sr+ (g) + e– E) Sr+2 (g) + 2e– → Sr (g) F) Sr (g) + e– → Sr– (g)arrow_forwardcan you help me with this question?arrow_forward
- Rank the following isoelectronic species in order of decreasing size: Argon atom (Ar), Calcium ion (Ca+2), chloride ion (Cl-1), Potassium ion (K+1), Sulfide ion (S-2).arrow_forwardTwo sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. ionization + F → F + e + Bi → Bi + e Te → Te + e energy required ? ? ? î ↑ ŷ ionization + PbPb te + CI + e Cl → Cl Sb Sb + e X energy required ? ? ? S ↑ ↑arrow_forwardTwo sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next lea energy, and so on. ionization Ar Ar+e Ba Ba +e Fr Fre energy required [? ? ? ionization F→ F + e Si Si + e Ra Ra +e X energy required ? ? ?arrow_forward
- Which has the largest radius? N3-, K+, Cl-, P3-, Sarrow_forwardTwo sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. energy energy ionization ionization required required olo + Sn с - с+е » Sn + e Ar Не — Не +e ? Cs Cs + e As - As +e ? Ga Ga + e ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY