
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
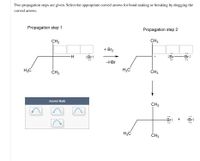
Transcribed Image Text:Two propagation steps are given. Select the appropriate curved arrows for bond making or breaking by dragging the
curved arrows.
Propagation step 1
Propagation step 2
CH3
CH3
+ Br2
:Br:
:Br
-Br:
-HBr
H3C
ČH3
H3C
CH3
Answer Bank
CH3
Br:
·Br:
H3C
ČH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- % Leaving Group, Tertiary Sketch and Submit Above Carbocation, NOS neutral nucleophile Addition (Sn1), Mono- Substituted Proton Transfer, Deprotonation, Base (conjugate acid) Carrier H₂C H₂C H,C-C H CH₂ CH₂ CH₂ CH₂ a OH CH₂ H3C CH3 Leaving Group, Tertiary + Br: H3C-C CH3 OH CH, Carbocation, NOS neutral nucleophile Addition (Sn1), Mono-Substituted CH₂ CH₂ Proton Transfer, Deprotonation, Base (conjugate acid) Carrier OH H₂C CH₂ Sketch and Submit Above xaxaarrow_forwardDRAW THE PROPAGATION STEPS WITH PROPER ELECTRON FLOW ARROWS FOR THE FOLLOWING REACTION. Br-Br Br H-Brarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. H HH Select to Add Arrows O..I H3O+ heat HH Select to Add Arrows H3O+ H heat I.O. Please select a drawing or reagent from the question areaarrow_forward
- Please give me idea why resonance is more reactive. I thought If resonance exists, it delocalizes more, and means less reactivearrow_forwardPlease don't provide handwriting solutionarrow_forwardA A c-) For each pair of resonance structures shown below (A and B), circle which resonance contributor makes a greater contribution to the resonance hybrid. Briefly explain your answer. O.. :0: B m N:arrow_forward
- j) Circle the л bond most likely to be hydrogenated in the following molecule. ( H2 ي هذهarrow_forward2) a) Provide Newman projects of the three staggered conformations of molecule 1 shown below looking down the indicated bond (1→2). Circle the most stable conformation. HH3C CH3 H molecule 1 b) Provide Newman projects of the three eclipsed conformations of molecule 1 looking down the indicated bond. Put a square around the least stable conformation.arrow_forwardMechanism: Show correct electron pushing formalism for the following reaction. Remember to show all intermediate structures. FeBr3 Br₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY